Fragment screening using capillary electrophoresis (CEfrag) for hit identification of heat shock protein 90 ATPase inhibitors.
Austin, C., Pettit, S.N., Magnolo, S.K., Sanvoisin, J., Chen, W., Wood, S.P., Freeman, L.D., Pengelly, R.J., Hughes, D.E.(2012) J Biomol Screen 17: 868-876
- PubMed: 22573733
- DOI: https://doi.org/10.1177/1087057112445785
- Primary Citation of Related Structures:
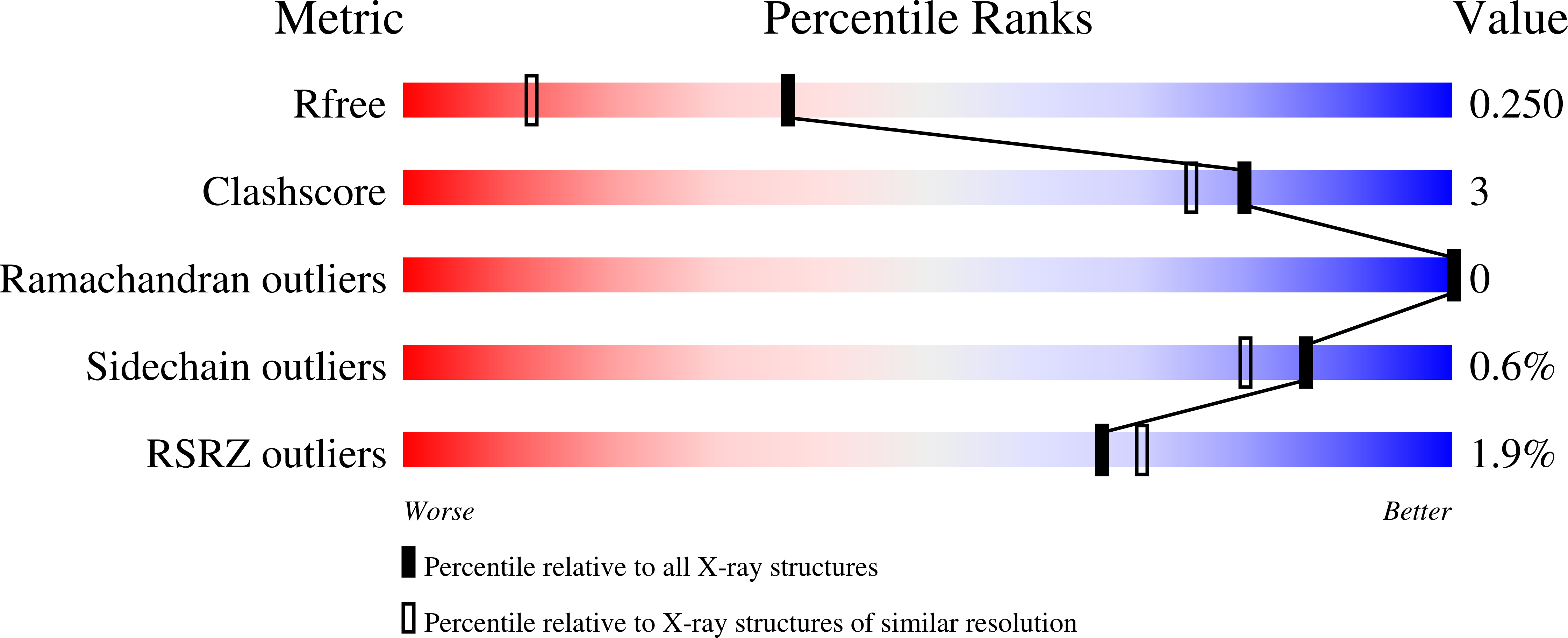
4EGH, 4EGI, 4EGK - PubMed Abstract:
CEfrag is a new fragment screening technology based on affinity capillary electrophoresis (ACE). Here we report on the development of a mobility shift competition assay using full-length human heat shock protein 90¦Á (Hsp90¦Á), radicicol as the competitor probe ligand, and successful screening of the Selcia fragment library. The CEfrag assay was able to detect weaker affinity (IC(50) >500 ?M) fragments than were detected by a fluorescence polarization competition assay using FITC-labeled geldanamycin. The binding site of selected fragments was determined by co-crystallization with recombinant Hsp90¦Á N-terminal domain and X-ray analysis. The results of this study confirm that CEfrag is a sensitive microscale technique enabling detection of fragments binding to the biological target in near-physiological solution.
Organizational Affiliation:
Discovery, Selcia Ltd, Fyfield Business and Research Park, Ongar, UK. carol.austin@selcia.com