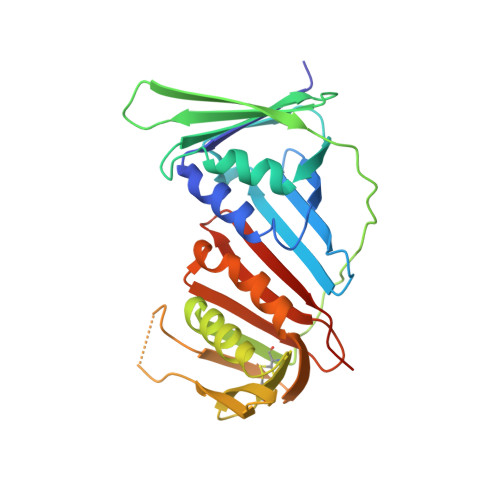
The p12 subunit of human polymerase delta uses an atypical PIP box for molecular recognition of proliferating cell nuclear antigen (PCNA).
Gonzalez-Magana, A., Ibanez de Opakua, A., Romano-Moreno, M., Murciano-Calles, J., Merino, N., Luque, I., Rojas, A.L., Onesti, S., Blanco, F.J., De Biasio, A.(2019) J Biological Chem 294: 3947-3956
- PubMed: 30655288
- DOI: https://doi.org/10.1074/jbc.RA118.006391
- Primary Citation of Related Structures:
6HVO - PubMed Abstract:
Human DNA polymerase ¦Ä is essential for DNA replication and acts in conjunction with the processivity factor proliferating cell nuclear antigen (PCNA). In addition to its catalytic subunit (p125), pol ¦Ä comprises three regulatory subunits (p50, p68, and p12). PCNA interacts with all of these subunits, but only the interaction with p68 has been structurally characterized. Here, we report solution NMR-, isothermal calorimetry-, and X-ray crystallography-based analyses of the p12-PCNA interaction, which takes part in the modulation of the rate and fidelity of DNA synthesis by pol ¦Ä. We show that p12 binds with micromolar affinity to the classical PIP-binding pocket of PCNA via a highly atypical PIP box located at the p12 N terminus. Unlike the canonical PIP box of p68, the PIP box of p12 lacks the conserved glutamine; binds through a 2-fork plug made of an isoleucine and a tyrosine residue at +3 and +8 positions, respectively; and is stabilized by an aspartate at +6 position, which creates a network of intramolecular hydrogen bonds. These findings add to growing evidence that PCNA can bind a diverse range of protein sequences that may be broadly grouped as PIP-like motifs as has been previously suggested.
Organizational Affiliation:
From the CIC bioGUNE, Parque Tecnol¨®gico de Bizkaia Edificio 800, 48160 Derio, Spain.