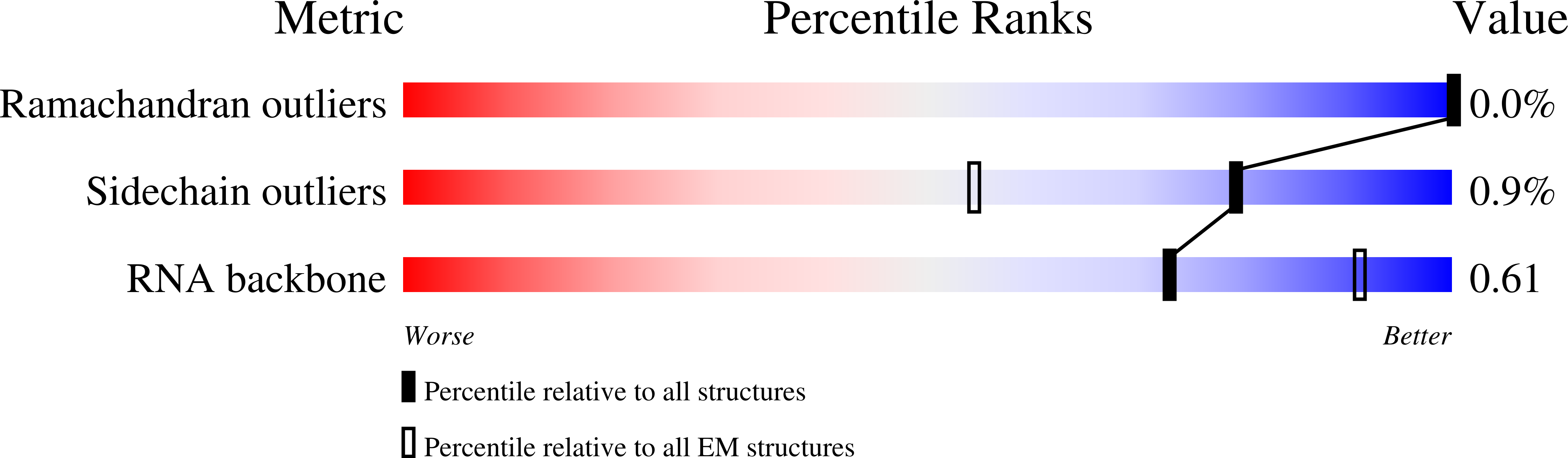Structures of the Staphylococcus aureus ribosome inhibited by fusidic acid and fusidic acid cyclopentane.
Gonzalez-Lopez, A., Larsson, D.S.D., Koripella, R.K., Cain, B.N., Chavez, M.G., Hergenrother, P.J., Sanyal, S., Selmer, M.(2024) Sci Rep 14: 14253-14253
- PubMed: 38902339
- DOI: https://doi.org/10.1038/s41598-024-64868-x
- Primary Citation of Related Structures:
8P2F, 8P2G, 8P2H - PubMed Abstract:
The antibiotic fusidic acid (FA) is used to treat Staphylococcus aureus infections. It inhibits protein synthesis by binding to elongation factor G (EF-G) and preventing its release from the ribosome after translocation. While FA, due to permeability issues, is only effective against gram-positive bacteria, the available structures of FA-inhibited complexes are from gram-negative model organisms. To fill this knowledge gap, we solved cryo-EM structures of the S. aureus ribosome in complex with mRNA, tRNA, EF-G and FA to 2.5?? resolution and the corresponding complex structures with the recently developed FA derivative FA-cyclopentane (FA-CP) to 2.0?? resolution. With both FA variants, the majority of the ribosomal particles are observed in chimeric state and only a minor population in post-translocational state. As expected, FA binds in a pocket between domains I, II and III of EF-G and the sarcin-ricin loop of 23S rRNA. FA-CP binds in an identical position, but its cyclopentane moiety provides additional contacts to EF-G and 23S rRNA, suggesting that its improved resistance profile towards mutations in EF-G is due to higher-affinity binding. These high-resolution structures reveal new details about the S. aureus ribosome, including confirmation of many rRNA modifications, and provide an optimal starting point for future structure-based drug discovery on an important clinical drug target.
Organizational Affiliation:
Department of Cell and Molecular Biology, Uppsala University, BMC, P.O. Box 596, 75124, Uppsala, Sweden.