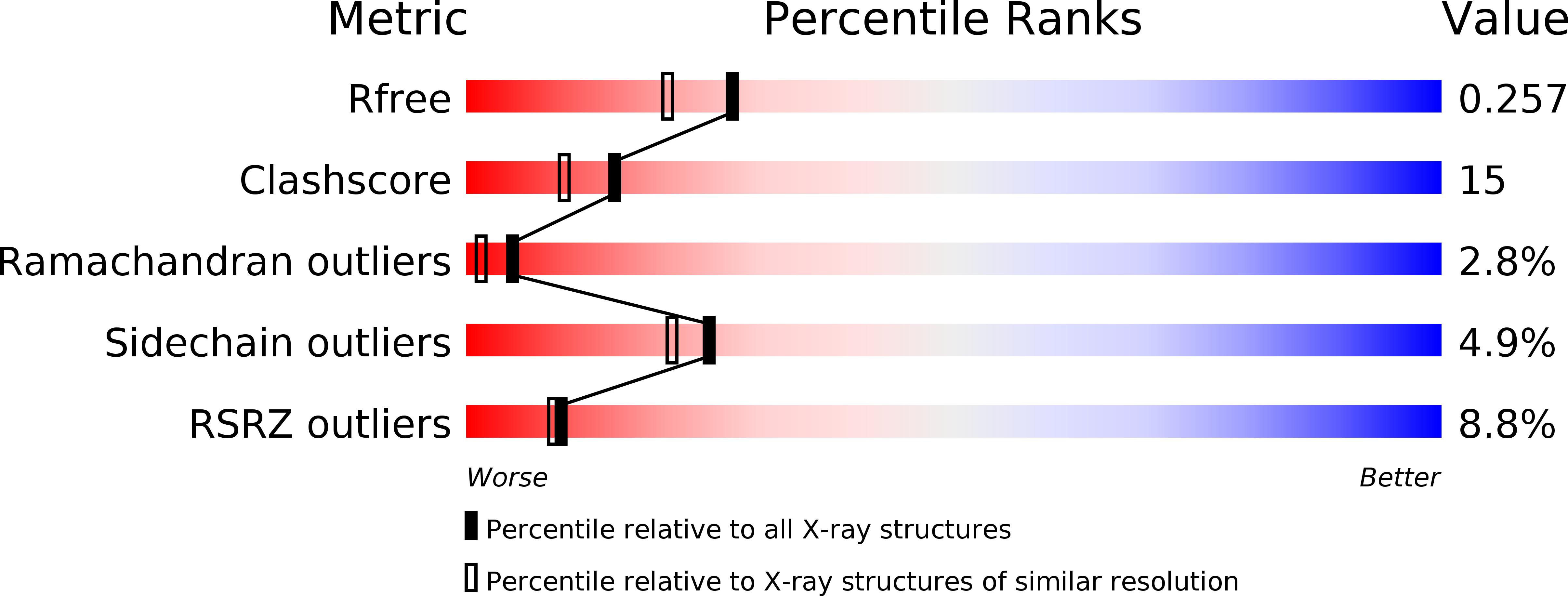
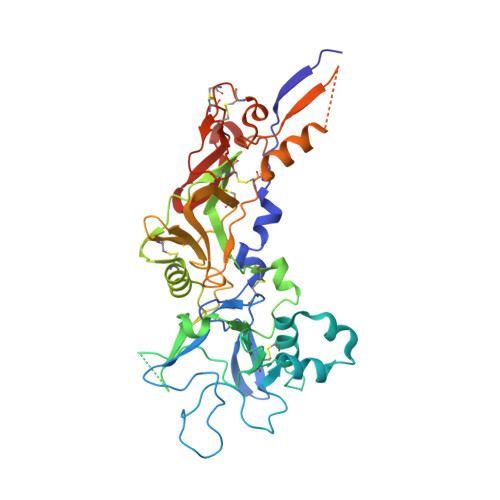
Crystal Structure of the Malaria Vaccine Candidate Apical Membrane Antigen 1
Pizarro, J.C., Vulliez-Le Normand, B., Chesne-Seck, M.-L., Collins, C., Withers-Martinez, C., Hackett, F., Blackman, M., Faber, B., Remarque, E., Kocken, C.H.M., Thomas, A.W., Bentley, G.A.(2005) Science 308: 408
- PubMed: 15731407
- DOI: https://doi.org/10.1126/science.1107449
- Primary Citation of Related Structures:
1W81, 1W8K - PubMed Abstract:
Apical membrane antigen 1 from Plasmodium is a leading malaria vaccine candidate. The protein is essential for host-cell invasion, but its molecular function is unknown. The crystal structure of the three domains comprising the ectoplasmic region of the antigen from P. vivax, solved at 1.8 angstrom resolution, shows that domains I and II belong to the PAN motif, which defines a superfamily of protein folds implicated in receptor binding. We also mapped the epitope of an invasion-inhibitory monoclonal antibody specific for the P. falciparum ortholog and modeled this to the structure. The location of the epitope and current knowledge on structure-function correlations for PAN domains together suggest a receptor-binding role during invasion in which domain II plays a critical part. These results are likely to aid vaccine and drug design.
Organizational Affiliation:
Unit¨¦ d'Immunologie Structurale, Centre National de la Recherche Scientifique, URA 2185, Institut Pasteur, 25 rue du Docteur Roux, 75724 Paris, France.