Radiation damage reveals promising interaction position
Koch, C., Heine, A., Klebe, G.(2011) J Synchrotron Radiat 18: 782-789
- PubMed: 21862860
- DOI: https://doi.org/10.1107/S0909049511027920
- Primary Citation of Related Structures:
3ONB, 3ONC - PubMed Abstract:
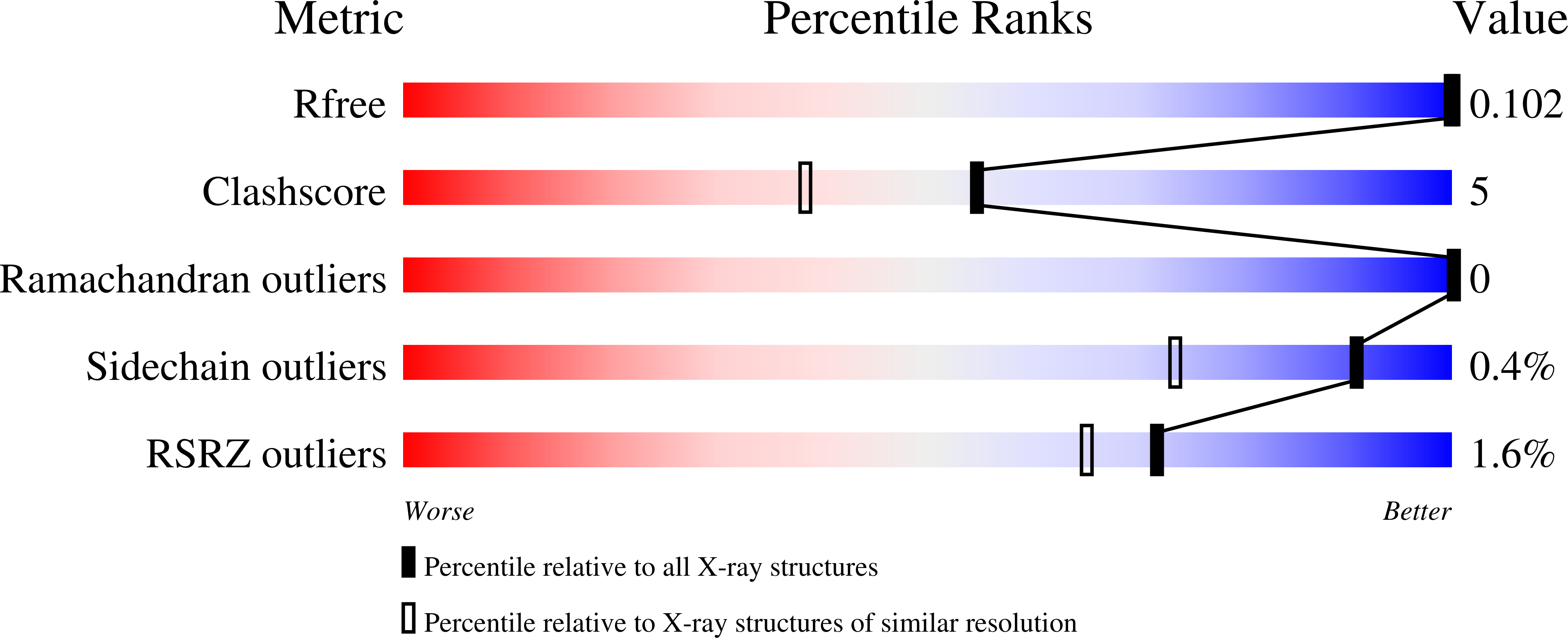
High-resolution structural data of protein inhibitor complexes are the key to rational drug design. Synchrotron radiation allows for atomic resolutions but is frequently accompanied by radiation damage to protein complexes. In this study a human aldose reductase mutant complexed with a bromine-substituted inhibitor was determined to atomic resolution [Protein Data Bank (PDB) code 3onc]. Though the radiation dose was moderate, a selective disruption of a bromine-inhibitor bond during the experiment was observed while the protein appears unaffected. A covalent bond to bromine is cleaved and the displaced atom is not scattered throughout the crystal but can most likely be assigned as a bromide to an additional difference electron density peak observed in the structure. The bromide relocates to an adjacent unoccupied site where promising interactions to protein residues stabilize its position. These findings were verified by a second similar structure determined with considerably higher radiation dose (PDB code 3onb).
Organizational Affiliation:
Philipps-Universit?t, Marburg, Germany.