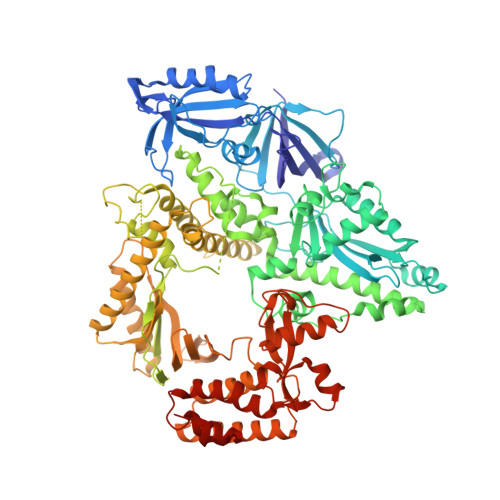
Activity and fidelity of human DNA polymerase alpha depend on primer structure.
Baranovskiy, A.G., Duong, V.N., Babayeva, N.D., Zhang, Y., Pavlov, Y.I., Anderson, K.S., Tahirov, T.H.(2018) J Biol Chem 293: 6824-6843
- PubMed: 29555682
- DOI: https://doi.org/10.1074/jbc.RA117.001074
- Primary Citation of Related Structures:
6AS7 - PubMed Abstract:
DNA polymerase ¦Á (Pol¦Á) plays an important role in genome replication. In a complex with primase, Pol¦Á synthesizes chimeric RNA-DNA primers necessary for replication of both chromosomal DNA strands. During RNA primer extension with deoxyribonucleotides, Pol¦Á needs to use double-stranded helical substrates having different structures. Here, we provide a detailed structure-function analysis of human Pol¦Á's interaction with dNTPs and DNA templates primed with RNA, chimeric RNA-DNA, or DNA. We report the crystal structures of two ternary complexes of the Pol¦Á catalytic domain containing dCTP, a DNA template, and either a DNA or an RNA primer. Unexpectedly, in the ternary complex with a DNA:DNA duplex and dCTP, the "fingers" subdomain of Pol¦Á is in the open conformation. Pol¦Á induces conformational changes in the DNA and hybrid duplexes to produce the universal double helix form. Pre-steady-state kinetic studies indicated for both duplex types that chemical catalysis rather than product release is the rate-limiting step. Moreover, human Pol¦Á extended DNA primers with higher efficiency but lower processivity than it did with RNA and chimeric primers. Pol¦Á has a substantial propensity to make errors during DNA synthesis, and we observed that its fidelity depends on the type of sugar at the primer 3'-end. A detailed structural comparison of Pol¦Á with other replicative DNA polymerases disclosed common features and some differences, which may reflect the specialization of each polymerase in genome replication.
Organizational Affiliation:
From the Eppley Institute for Research in Cancer and Allied Diseases, Fred and Pamela Buffett Cancer Center, and.