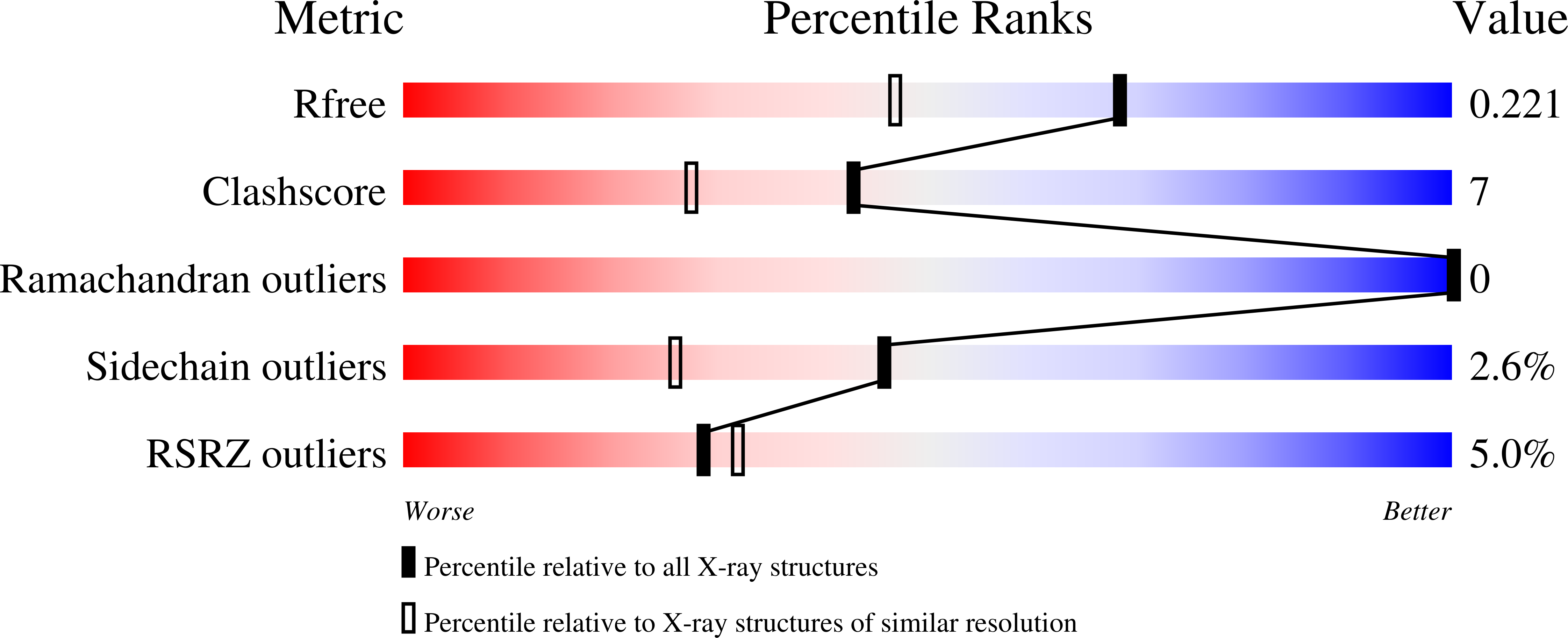
Structure of the RuBisCO chaperone RbcX from the thermophilic cyanobacterium Thermosynechococcus elongatus
Tarnawski, M., Krzywda, S., Bialek, W., Jaskolski, M., Szczepaniak, A.(2011) Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 851-857
- PubMed: 21821880
- DOI: https://doi.org/10.1107/S1744309111018860
- Primary Citation of Related Structures:
3Q20 - PubMed Abstract:
The crystal structure of TeRbcX, a RuBisCO assembly chaperone from the cyanobacterium Thermosynechococcus elongatus, a thermophilic organism, has been determined at 1.7?? resolution. TeRbcX has an unusual cysteine residue at position 103 that is not found in RbcX proteins from mesophilic organisms. Unlike wild-type TeRbcX, a mutant protein with Cys103 replaced by Ala (TeRbcX-C103A) could be readily crystallized. The structure revealed that the overall fold of the TeRbcX homodimer is similar to those of previously crystallized RbcX proteins. Normal-mode analysis suggested that TeRbcX might adopt an open or closed conformation through a hinge movement pivoted on a kink in two long ¦Á4 helices. This type of conformational transition is presumably connected to RbcL (the large RuBisCO subunit) binding during the chaperone function of the RuBisCO assembly.
Organizational Affiliation:
Department of Biophysics, Faculty of Biotechnology, University of Wroclaw, Wroclaw, Poland.