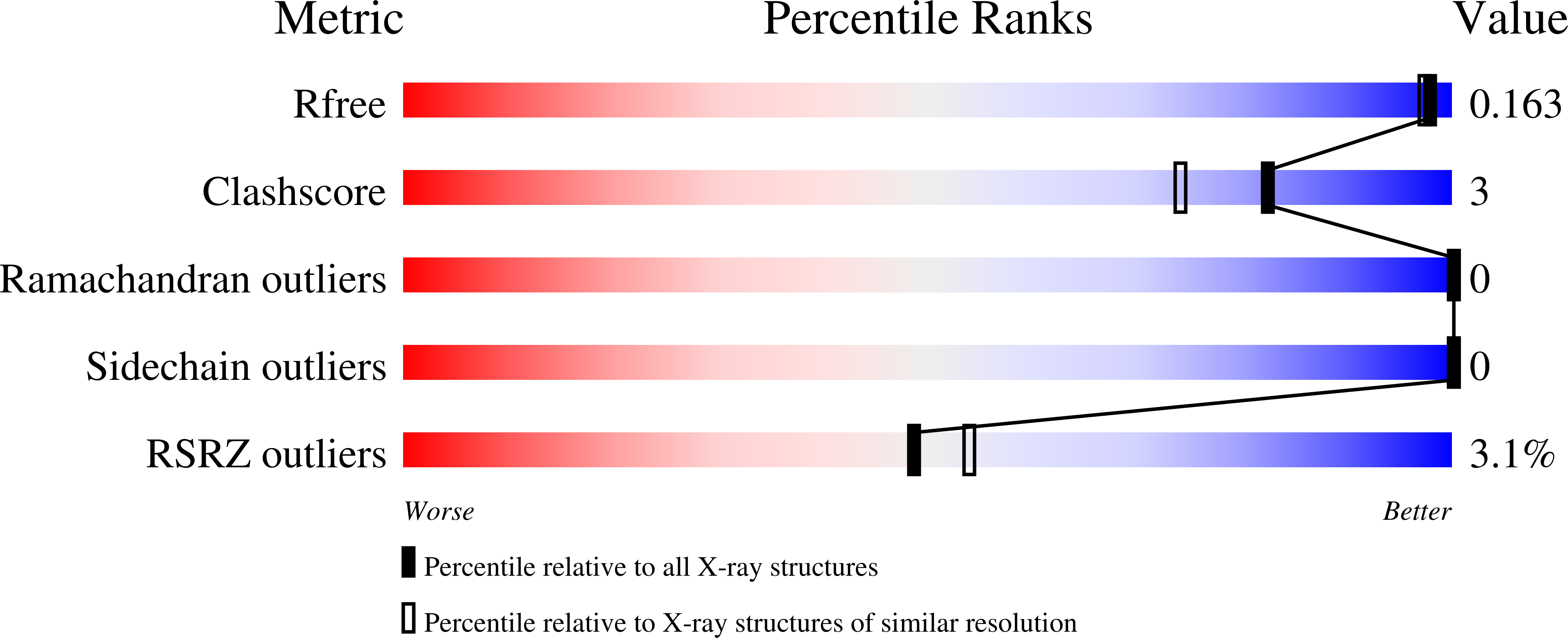
A small-molecule mimic of a peptide docking motif inhibits the protein kinase PDK1.
Rettenmaier, T.J., Sadowsky, J.D., Thomsen, N.D., Chen, S.C., Doak, A.K., Arkin, M.R., Wells, J.A.(2014) Proc Natl Acad Sci U S A 111: 18590-18595
- PubMed: 25518860
- DOI: https://doi.org/10.1073/pnas.1415365112
- Primary Citation of Related Structures:
4RQK, 4RQV, 4RRV - PubMed Abstract:
There is great interest in developing selective protein kinase inhibitors by targeting allosteric sites, but these sites often involve protein-protein or protein-peptide interfaces that are very challenging to target with small molecules. Here we present a systematic approach to targeting a functionally conserved allosteric site on the protein kinase PDK1 called the PDK1-interacting fragment (PIF)tide-binding site, or PIF pocket. More than two dozen prosurvival and progrowth kinases dock a conserved peptide tail into this binding site, which recruits them to PDK1 to become activated. Using a site-directed chemical screen, we identified and chemically optimized ligand-efficient, selective, and cell-penetrant small molecules (molecular weight ¡« 380 Da) that compete with the peptide docking motif for binding to PDK1. We solved the first high-resolution structure of a peptide docking motif (PIFtide) bound to PDK1 and mapped binding energy hot spots using mutational analysis. We then solved structures of PDK1 bound to the allosteric small molecules, which revealed a binding mode that remarkably mimics three of five hot-spot residues in PIFtide. These allosteric small molecules are substrate-selective PDK1 inhibitors when used as single agents, but when combined with an ATP-competitive inhibitor, they completely suppress the activation of the downstream kinases. This work provides a promising new scaffold for the development of high-affinity PIF pocket ligands, which may be used to enhance the anticancer activity of existing PDK1 inhibitors. Moreover, our results provide further impetus for exploring the helix ¦ÁC patches of other protein kinases as potential therapeutic targets even though they involve protein-protein interfaces.
Organizational Affiliation:
Chemistry and Chemical Biology Graduate Program, Department of Pharmaceutical Chemistry.