Crystallographic insights into the structure-activity relationships of diazaborine enoyl-ACP reductase inhibitors.
Jordan, C.A., Sandoval, B.A., Serobyan, M.V., Gilling, D.H., Groziak, M.P., Xu, H.H., Vey, J.L.(2015) Acta Crystallogr F Struct Biol Commun 71: 1521-1530
- PubMed: 26625295
- DOI: https://doi.org/10.1107/S2053230X15022098
- Primary Citation of Related Structures:
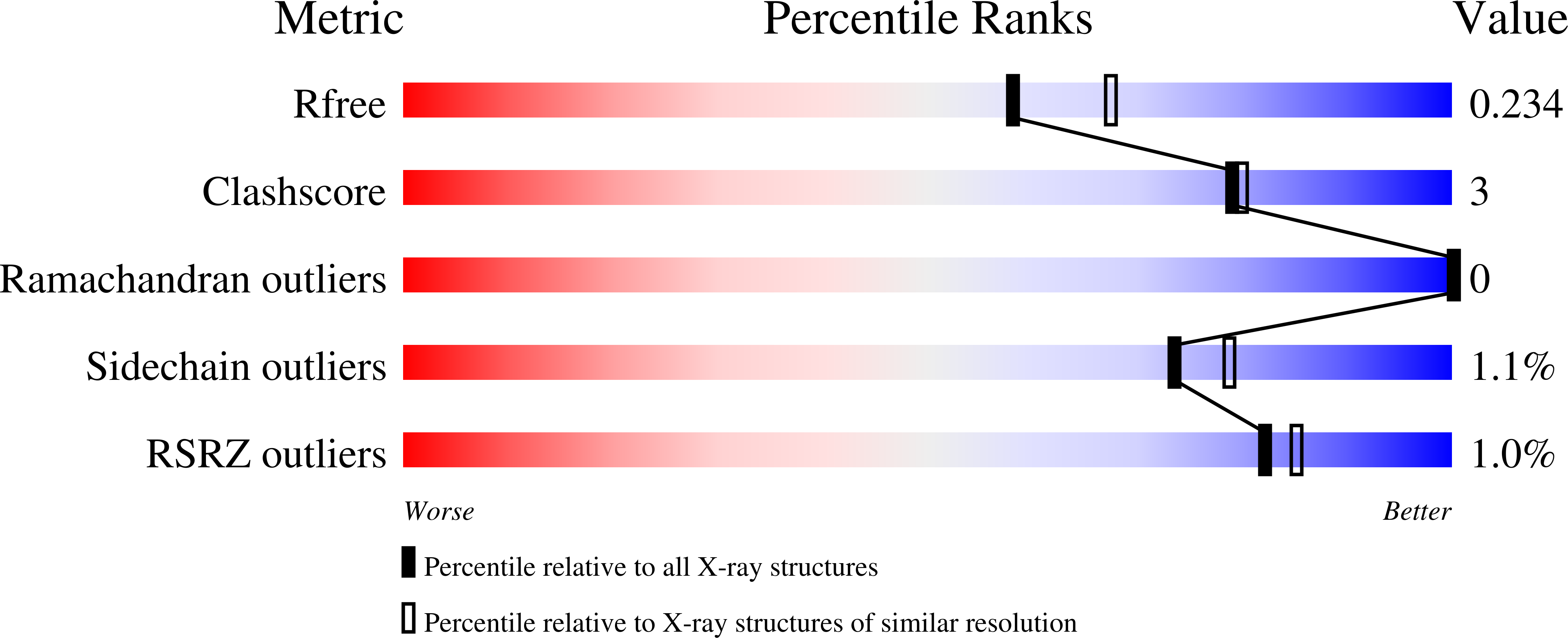
5CFZ, 5CG1, 5CG2 - PubMed Abstract:
Enoyl-ACP reductase, the last enzyme of the fatty-acid biosynthetic pathway, is the molecular target for several successful antibiotics such as the tuberculosis therapeutic isoniazid. It is currently under investigation as a narrow-spectrum antibiotic target for the treatment of several types of bacterial infections. The diazaborine family is a group of boron heterocycle-based synthetic antibacterial inhibitors known to target enoyl-ACP reductase. Development of this class of molecules has thus far focused solely on the sulfonyl-containing versions. Here, the requirement for the sulfonyl group in the diazaborine scaffold was investigated by examining several recently characterized enoyl-ACP reductase inhibitors that lack the sulfonyl group and exhibit additional variability in substitutions, size and flexibility. Biochemical studies are reported showing the inhibition of Escherichia coli enoyl-ACP reductase by four diazaborines, and the crystal structures of two of the inhibitors bound to E. coli enoyl-ACP reductase solved to 2.07 and 2.11?? resolution are reported. The results show that the sulfonyl group can be replaced with an amide or thioamide without disruption of the mode of inhibition of the molecule.
Organizational Affiliation:
Department of Chemistry and Biochemistry, California State University Northridge, Northridge, CA 91330-8262, USA.