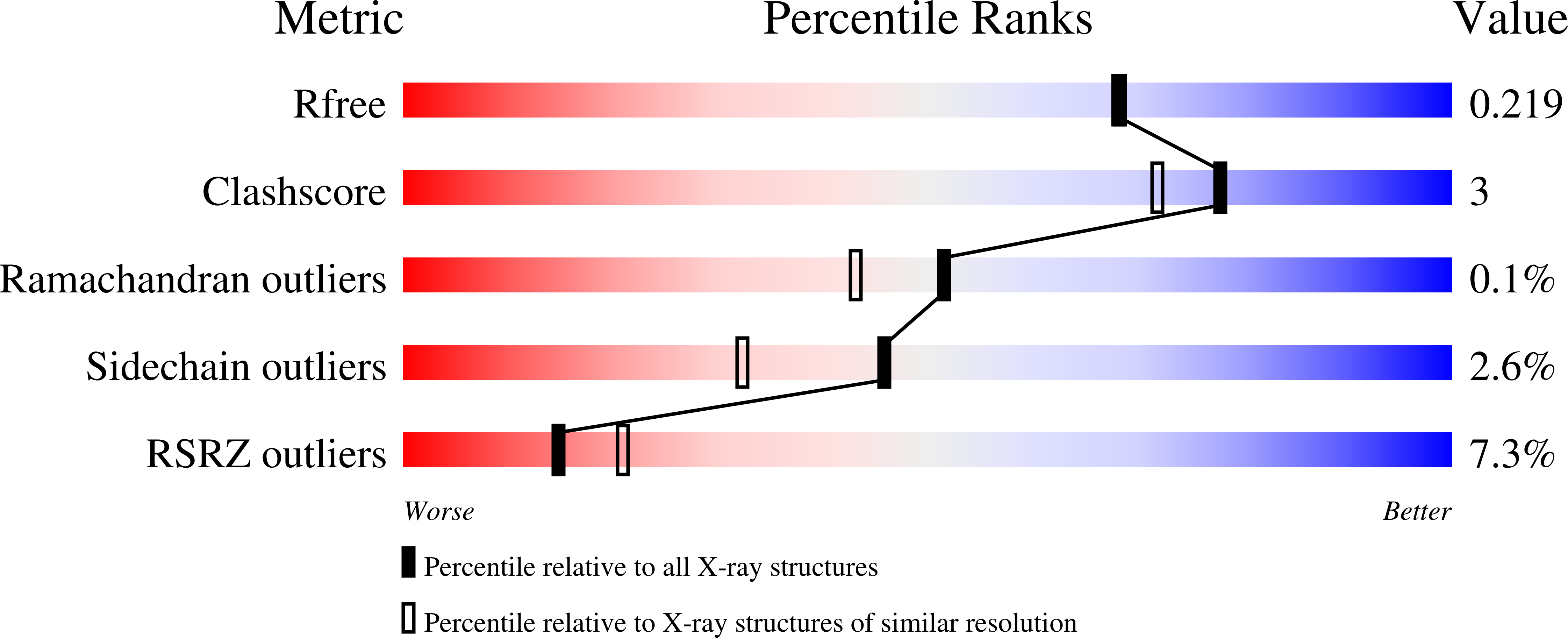
In crystallo screening for proline analog inhibitors of the proline cycle enzyme PYCR1.
Christensen, E.M., Bogner, A.N., Vandekeere, A., Tam, G.S., Patel, S.M., Becker, D.F., Fendt, S.M., Tanner, J.J.(2020) J Biological Chem 295: 18316-18327
- PubMed: 33109600
- DOI: https://doi.org/10.1074/jbc.RA120.016106
- Primary Citation of Related Structures:
6XOZ, 6XP0, 6XP1, 6XP2, 6XP3 - PubMed Abstract:
Pyrroline-5-carboxylate reductase 1 (PYCR1) catalyzes the biosynthetic half-reaction of the proline cycle by reducing ¦¤ 1 -pyrroline-5-carboxylate (P5C) to proline through the oxidation of NAD(P)H. Many cancers alter their proline metabolism by up-regulating the proline cycle and proline biosynthesis, and knockdowns of PYCR1 lead to decreased cell proliferation. Thus, evidence is growing for PYCR1 as a potential cancer therapy target. Inhibitors of cancer targets are useful as chemical probes for studying cancer mechanisms and starting compounds for drug discovery; however, there is a notable lack of validated inhibitors for PYCR1. To fill this gap, we performed a small-scale focused screen of proline analogs using X-ray crystallography. Five inhibitors of human PYCR1 were discovered: l-tetrahydro-2-furoic acid, cyclopentanecarboxylate, l-thiazolidine-4-carboxylate, l-thiazolidine-2-carboxylate, and N -formyl l-proline (NFLP). The most potent inhibitor was NFLP, which had a competitive (with P5C) inhibition constant of 100 ¦̀m The structure of PYCR1 complexed with NFLP shows that inhibitor binding is accompanied by conformational changes in the active site, including the translation of an ¦Á-helix by 1 ?. These changes are unique to NFLP and enable additional hydrogen bonds with the enzyme. NFLP was also shown to phenocopy the PYCR1 knockdown in MCF10A H-RAS V12 breast cancer cells by inhibiting de novo proline biosynthesis and impairing spheroidal growth. In summary, we generated the first validated chemical probe of PYCR1 and demonstrated proof-of-concept for screening proline analogs to discover inhibitors of the proline cycle.
Organizational Affiliation:
Department of Chemistry, University of Missouri, Columbia, Missouri, USA.