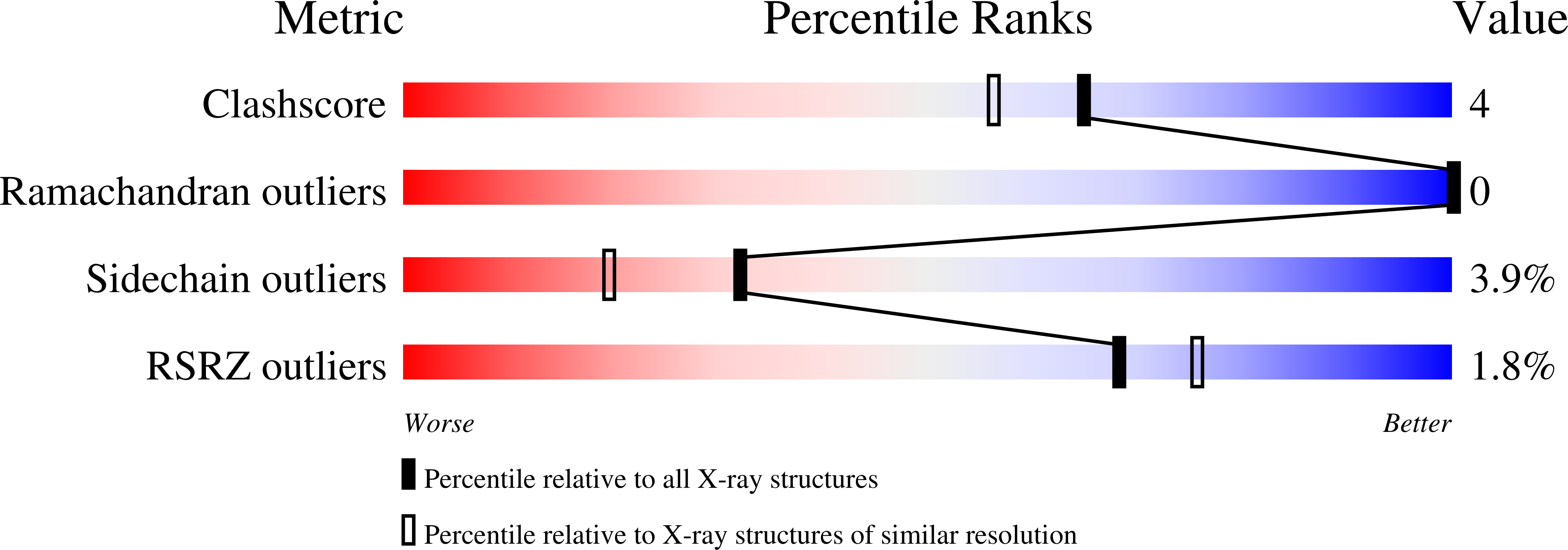
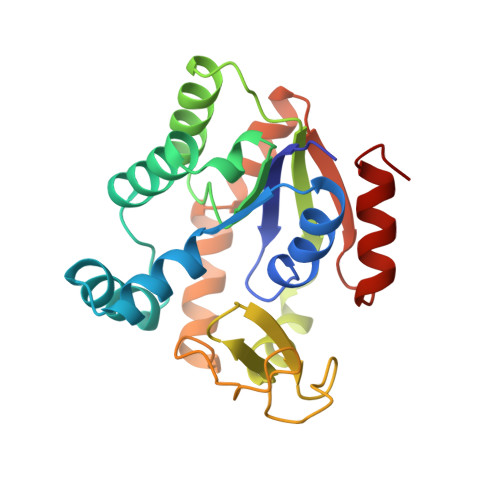
High-resolution structures of adenylate kinase from yeast ligated with inhibitor Ap5A, showing the pathway of phosphoryl transfer.
Abele, U., Schulz, G.E.(1995) Protein Sci 4: 1262-1271
- PubMed: 7670369
- DOI: https://doi.org/10.1002/pro.5560040702
- Primary Citation of Related Structures:
1AKY, 2AKY - PubMed Abstract:
The structure of adenylate kinase from yeast ligated with the two-substrate-mimicking inhibitor Ap5A and Mg2+ has been refined to 1.96 A resolution. In addition, the refined structure of the same complex with a bound imidazole molecule replacing Mg2+ has been determined at 1.63 A. These structures indicate that replacing Mg2+ by imidazole disturbs the water structure and thus the complex. A comparison with the G-proteins shows that Mg2+ is exactly at the same position with respect to the phosphates. However, although the Mg2+ ligand sphere of the G-proteins is a regular octahedron containing peptide ligands, the reported adenylate kinase has no such ligands and an open octahedron leaving space for the Mg2+ to accompany the transferred phosphoryl group. A superposition of the known crystalline and therefore perturbed phosphoryl transfer geometries in the adenylate kinases demonstrates that all of them are close to the start of the forward reaction with bound ATP and AMP. Averaging all observed perturbed structures gives rise to a close approximation of the transition state, indicating in general how to establish an elusive transition state geometry. The average shows that the in-line phosphoryl transfer is associative, because there is no space for a dissociative metaphosphate intermediate. As a side result, the secondary dipole interaction in the alpha-helices of both protein structures has been quantified.
Organizational Affiliation:
Institut f¨¹r Organische Chemie und Biochemie, Albert-Ludwigs-Universit?t, Freiburg im Breisgau, Germany.