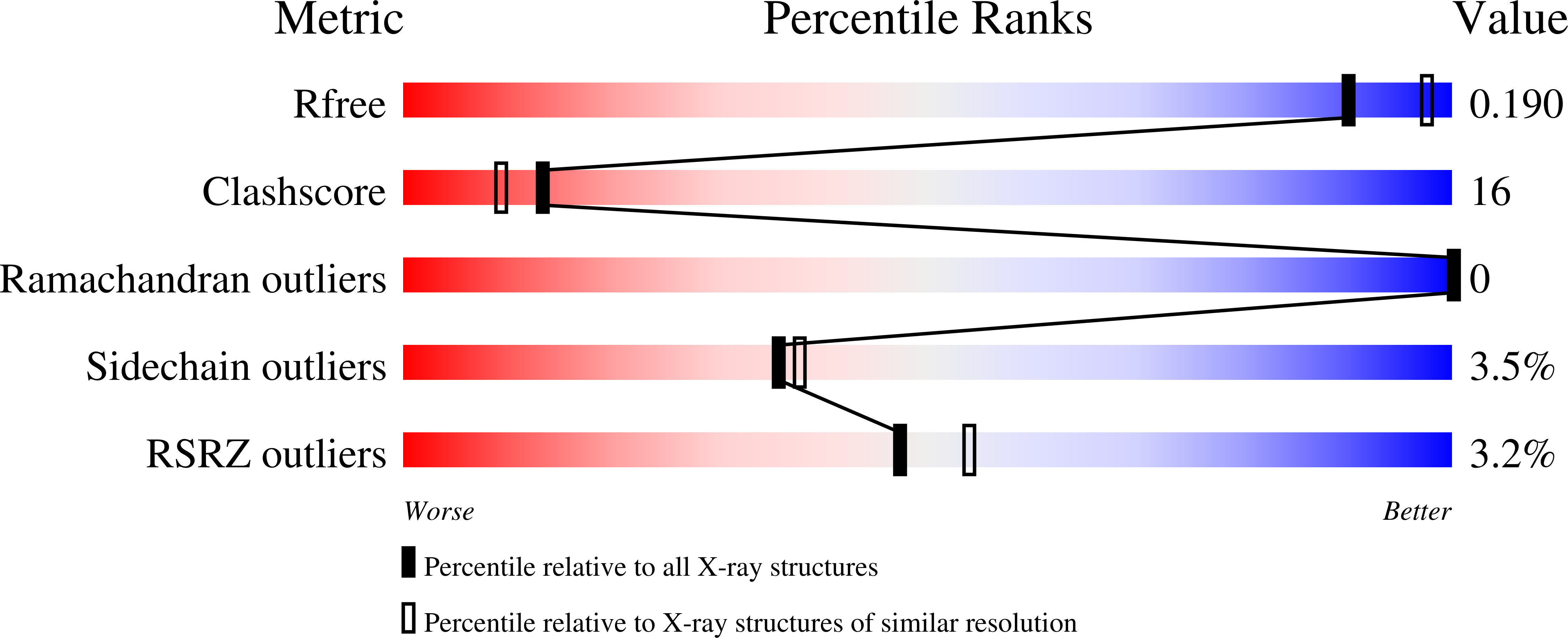
The Crystal Structure of TREX1 Explains the 3' Nucleotide Specificity and Reveals a Polyproline II Helix for Protein Partnering.
de Silva, U., Choudhury, S., Bailey, S.L., Harvey, S., Perrino, F.W., Hollis, T.(2007) J Biol Chem 282: 10537-10543
- PubMed: 17293595
- DOI: https://doi.org/10.1074/jbc.M700039200
- Primary Citation of Related Structures:
2IOC, 2OA8 - PubMed Abstract:
The TREX1 enzyme processes DNA ends as the major 3' --> 5' exonuclease activity in human cells. Mutations in the TREX1 gene are an underlying cause of the neurological brain disease Aicardi-Gouti¨¨res syndrome implicating TREX1 dysfunction in an aberrant immune response. TREX1 action during apoptosis likely prevents autoimmune reaction to DNA that would otherwise persist. To understand the impact of TREX1 mutations identified in patients with Aicardi-Gouti¨¨res syndrome on structure and activity we determined the x-ray crystal structure of the dimeric mouse TREX1 protein in substrate and product complexes containing single-stranded DNA and deoxyadenosine monophosphate, respectively. The structures show the specific interactions between the bound nucleotides and the residues lining the binding pocket of the 3' terminal nucleotide within the enzyme active site that account for specificity, and provide the molecular basis for understanding mutations that lead to disease. Three mutant forms of TREX1 protein identified in patients with Aicardi-Gouti¨¨res syndrome were prepared and the measured activities show that these specific mutations reduce enzyme activity by 4-35,000-fold. The structure also reveals an 8-amino acid polyproline II helix within the TREX1 enzyme that suggests a mechanism for interactions of this exonuclease with other protein complexes.
Organizational Affiliation:
Department of Biochemistry, Center for Structural Biology, Wake Forest University Health Sciences, Winston-Salem, North Carolina 27157.