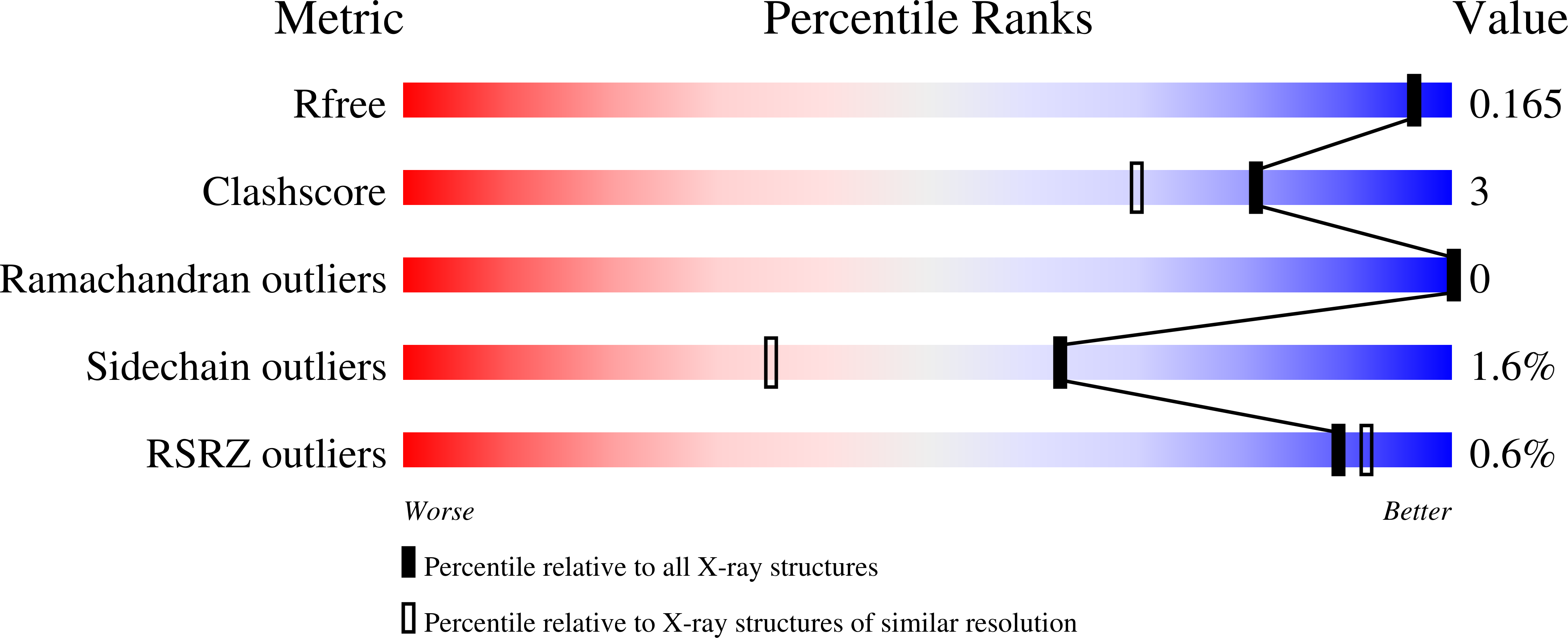
Displacement of disordered water molecules from hydrophobic pocket creates enthalpic signature: binding of phosphonamidate to the S1'-pocket of thermolysin.
Englert, L., Biela, A., Zayed, M., Heine, A., Hangauer, D., Klebe, G.(2010) Biochim Biophys Acta 1800: 1192-1202
- PubMed: 20600625
- DOI: https://doi.org/10.1016/j.bbagen.2010.06.009
- Primary Citation of Related Structures:
3FLF, 3FV4 - PubMed Abstract:
Prerequisite for the design of tight binding protein inhibitors and prediction of their properties is an in-depth understanding of the structural and thermodynamic details of the binding process. A series of closely related phosphonamidates was studied to elucidate the forces underlying their binding affinity to thermolysin. The investigated inhibitors are identical except for the parts penetrating into the hydrophobic S?'-pocket. A correlation of structural, kinetic and thermodynamic data was carried out by X-ray crystallography, kinetic inhibition assay and isothermal titration calorimetry. Binding affinity increases with larger ligand hydrophobic P?'-moieties accommodating the S?'-pocket. Surprisingly, larger P?'-side chain modifications are accompanied by an increase in the enthalpic contribution to binding. In agreement with other studies, it is suggested that the release of largely disordered waters from an imperfectly hydrated pocket results in an enthalpically favourable integration of these water molecules into bulk water upon inhibitor binding. This enthalpically favourable process contributes more strongly to the binding energetics than the entropy increase resulting from the release of water molecules from the S?'-pocket or the formation of apolar interactions between protein and inhibitor. Displacement of highly disordered water molecules from a rather imperfectly hydrated and hydrophobic specificity pocket can reveal an enthalpic signature of inhibitor binding.
Organizational Affiliation:
Department of Pharmaceutical Chemistry, Philipps-University Marburg, 35032 Marburg, Germany.