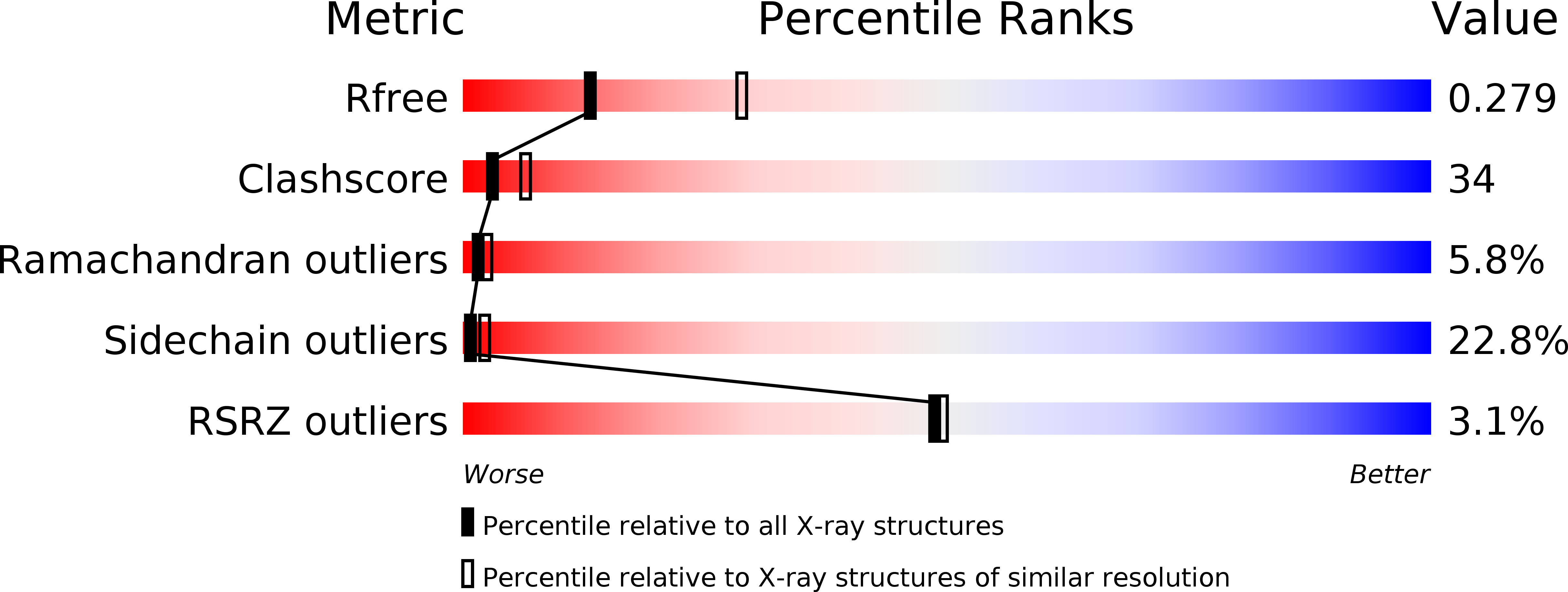
Structural characterization of proline-rich tyrosine kinase 2 (PYK2) reveals a unique (DFG-out) conformation and enables inhibitor design.
Han, S., Mistry, A., Chang, J.S., Cunningham, D., Griffor, M., Bonnette, P.C., Wang, H., Chrunyk, B.A., Aspnes, G.E., Walker, D.P., Brosius, A.D., Buckbinder, L.(2009) J Biological Chem 284: 13193-13201
- PubMed: 19244237
- DOI: https://doi.org/10.1074/jbc.M809038200
- Primary Citation of Related Structures:
3FZO, 3FZP, 3FZR, 3FZS, 3FZT - PubMed Abstract:
Proline-rich tyrosine kinase 2 (PYK2) is a cytoplasmic, non-receptor tyrosine kinase implicated in multiple signaling pathways. It is a negative regulator of osteogenesis and considered a viable drug target for osteoporosis treatment. The high-resolution structures of the human PYK2 kinase domain with different inhibitor complexes establish the conventional bilobal kinase architecture and show the conformational variability of the DFG loop. The basis for the lack of selectivity for the classical kinase inhibitor, PF-431396, within the FAK family is explained by our structural analyses. Importantly, the novel DFG-out conformation with two diarylurea inhibitors (BIRB796, PF-4618433) reveals a distinct subclass of non-receptor tyrosine kinases identifiable by the gatekeeper Met-502 and the unique hinge loop conformation of Leu-504. This is the first example of a leucine residue in the hinge loop that blocks the ATP binding site in the DFG-out conformation. Our structural, biophysical, and pharmacological studies suggest that the unique features of the DFG motif, including Leu-504 hinge-loop variability, can be exploited for the development of selective protein kinase inhibitors.
Organizational Affiliation:
Pfizer Global Research & Development, Groton, CT 06340, USA. seungil.han@pfizer.com