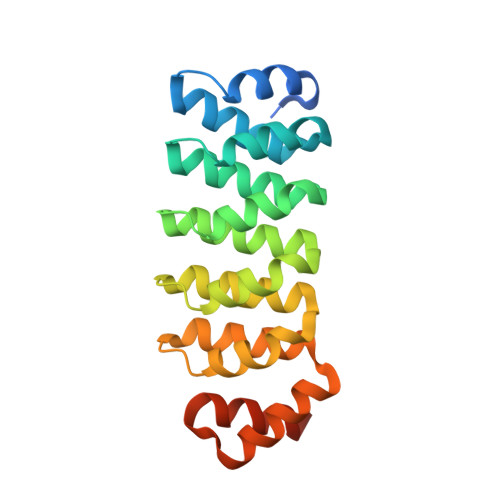
Design, production and molecular structure of a new family of artificial alpha-helicoidal repeat proteins ( alpha Rep) based on thermostable HEAT-like repeats
Urvoas, A., Guellouz, A., Valerio-Lepiniec, M., Graille, M., Durand, D., Desravines, D.C., van Tilbeurgh, H., Desmadril, M., Minard, P.(2010) J Mol Biology 404: 307-327
- PubMed: 20887736
- DOI: https://doi.org/10.1016/j.jmb.2010.09.048
- Primary Citation of Related Structures:
3LTJ, 3LTM - PubMed Abstract:
Repeat proteins have a modular organization and a regular architecture that make them attractive models for design and directed evolution experiments. HEAT repeat proteins, although very common, have not been used as a scaffold for artificial proteins, probably because they are made of long and irregular repeats. Here, we present and validate a consensus sequence for artificial HEAT repeat proteins. The sequence was defined from the structure-based sequence analysis of a thermostable HEAT-like repeat protein. Appropriate sequences were identified for the N- and C-caps. A library of genes coding for artificial proteins based on this sequence design, named ¦ÁRep, was assembled using new and versatile methodology based on circular amplification. Proteins picked randomly from this library are expressed as soluble proteins. The biophysical properties of proteins with different numbers of repeats and different combinations of side chains in hypervariable positions were characterized. Circular dichroism and differential scanning calorimetry experiments showed that all these proteins are folded cooperatively and are very stable (T(m) >70?ˇăC). Stability of these proteins increases with the number of repeats. Detailed gel filtration and small-angle X-ray scattering studies showed that the purified proteins form either monomers or dimers. The X-ray structure of a stable dimeric variant structure was solved. The protein is folded with a highly regular topology and the repeat structure is organized, as expected, as pairs of alpha helices. In this protein variant, the dimerization interface results directly from the variable surface enriched in aromatic residues located in the randomized positions of the repeats. The dimer was crystallized both in an apo and in a PEG-bound form, revealing a very well defined binding crevice and some structure flexibility at the interface. This fortuitous binding site could later prove to be a useful binding site for other low molecular mass partners.
Organizational Affiliation:
Institut de Biochimie Mol¨¦culaire et Cellulaire (IBBMC), Univ Paris Sud, UMR 8619, Orsay, F-91405 Orsay, France.