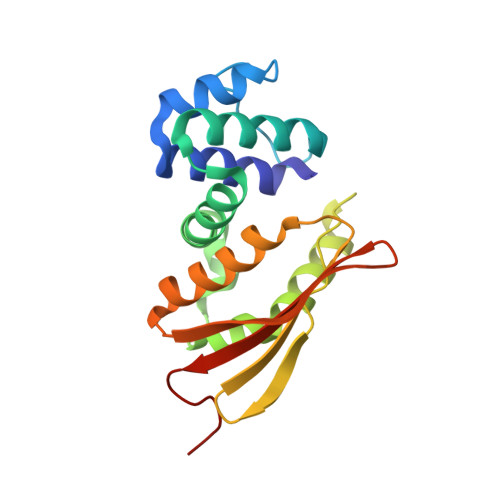
Ru-porphyrin protein scaffolds for sensing O2.
Winter, M.B., McLaurin, E.J., Reece, S.Y., Olea, C., Nocera, D.G., Marletta, M.A.(2010) J Am Chem Soc 132: 5582-5583
- PubMed: 20373741
- DOI: https://doi.org/10.1021/ja101527r
- Primary Citation of Related Structures:
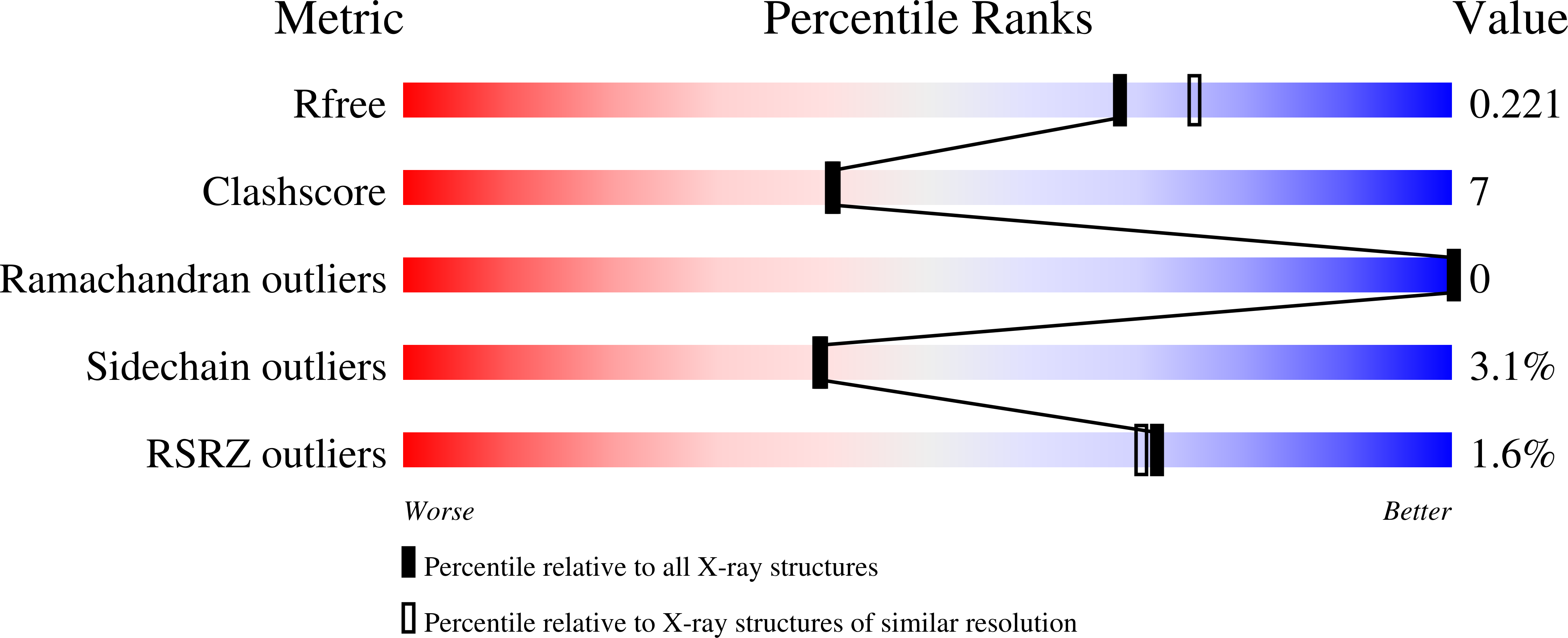
3M0B - PubMed Abstract:
Hemoprotein-based scaffolds containing phosphorescent ruthenium(II) CO mesoporphyrin IX (RuMP) are reported here for oxygen (O(2)) sensing in biological contexts. RuMP was incorporated into the protein scaffolds during protein expression utilizing a novel method that we have described previously. A high-resolution (2.00 A) crystal structure revealed that the unnatural porphyrin binds to the proteins in a manner similar to the native heme and does not perturb the protein fold. The protein scaffolds were found to provide unique coordination environments for RuMP and modulate the porphyrin emission properties. Emission lifetime measurements demonstrate a linear O(2) response within the physiological range and precision comparable to commercial O(2) sensors. The RuMP proteins are robust, readily modifiable platforms and display promising O(2) sensing properties for future in vivo applications.
Organizational Affiliation:
Department of Chemistry, QB3 Institute, and Division of Physical Biosciences, Lawrence Berkeley National Laboratory, University of California, Berkeley, California 94720-3220, USA.