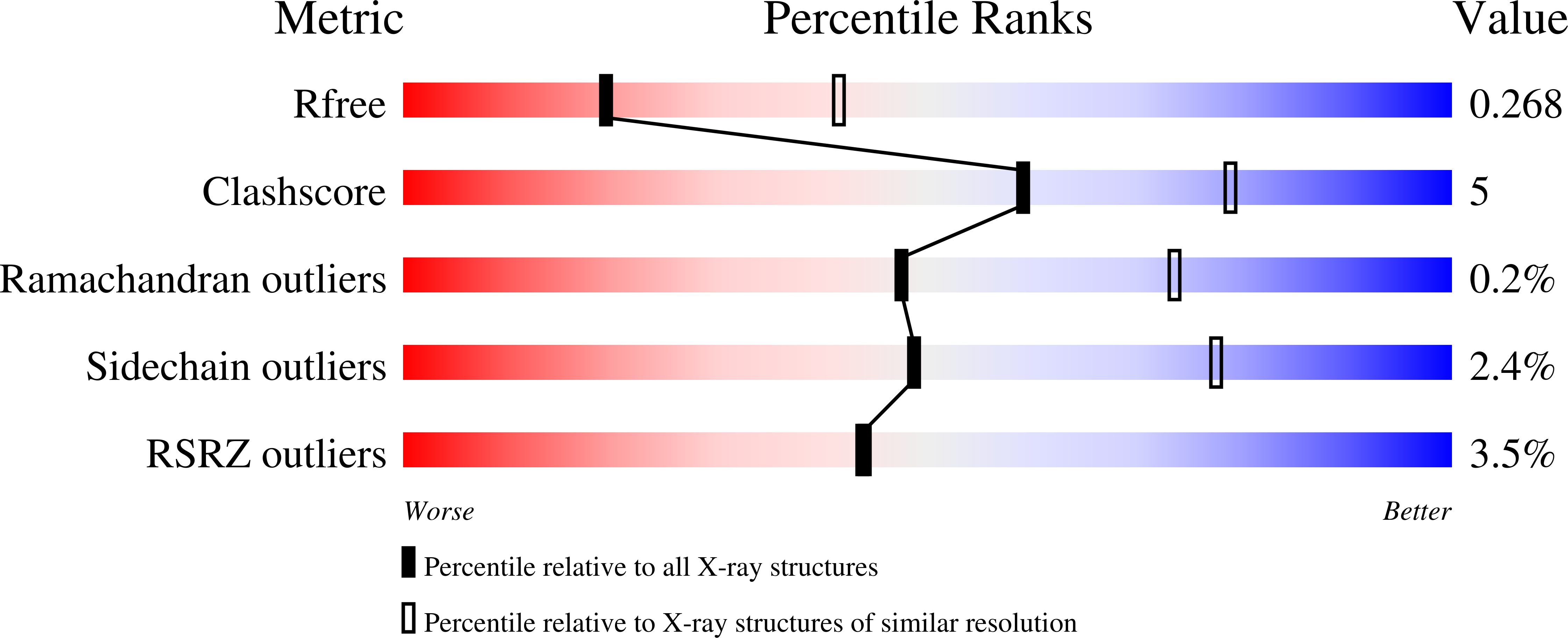
Substitution for Asn460 cripples {beta}-galactosidase (Escherichia coli) by increasing substrate affinity and decreasing transition state stability.
Wheatley, R.W., Kappelhoff, J.C., Hahn, J.N., Dugdale, M.L., Dutkoski, M.J., Tamman, S.D., Fraser, M.E., Huber, R.E.(2012) Arch Biochem Biophys 521: 51-61
- PubMed: 22446164
- DOI: https://doi.org/10.1016/j.abb.2012.03.014
- Primary Citation of Related Structures:
3VD3, 3VD4, 3VD5, 3VD7, 3VD9, 3VDA, 3VDB, 3VDC - PubMed Abstract:
Substrate initially binds to ¦Â-galactosidase (Escherichia coli) at a 'shallow' site. It then moves ¡«3? to a 'deep' site and the transition state forms. Asn460 interacts in both sites, forming a water bridge interaction with the O3 hydroxyl of the galactosyl moiety in the shallow site and a direct H-bond with the O2 hydroxyl of the transition state in the deep site. Structural and kinetic studies were done with ¦Â-galactosidases with substitutions for Asn460. The substituted enzymes have enhanced substrate affinity in the shallow site indicating lower E¡¤substrate complex energy levels. They have poor transition state stabilization in the deep site that is manifested by increased energy levels of the E¡¤transition state complexes. These changes in stability result in increased activation energies and lower k(cat) values. Substrate affinity to N460D-¦Â-galactosidase was enhanced through greater binding enthalpy (stronger H-bonds through the bridging water) while better affinity to N460T-¦Â-galactosidase occurred because of greater binding entropy. The transition states are less stable with N460S- and N460T-¦Â-galactosidase because of the weakening or loss of the important bond to the O2 hydroxyl of the transition state. For N460D-¦Â-galactosidase, the transition state is less stable due to an increased entropy penalty.
Organizational Affiliation:
Division of Biochemistry, Faculty of Science, University of Calgary, Calgary, AB, Canada T2N 1N4.