An Improved Racemase/Acylase Biotransformation for the Preparation of Enantiomerically Pure Amino Acids.
Baxter, S., Royer, S., Grogan, G., Brown, F., Holt-Tiffin, K.E., Taylor, I.N., Fotheringham, I.G., Campopiano, D.J.(2012) J Am Chem Soc 134: 19310
- PubMed: 23130969
- DOI: https://doi.org/10.1021/ja305438y
- Primary Citation of Related Structures:
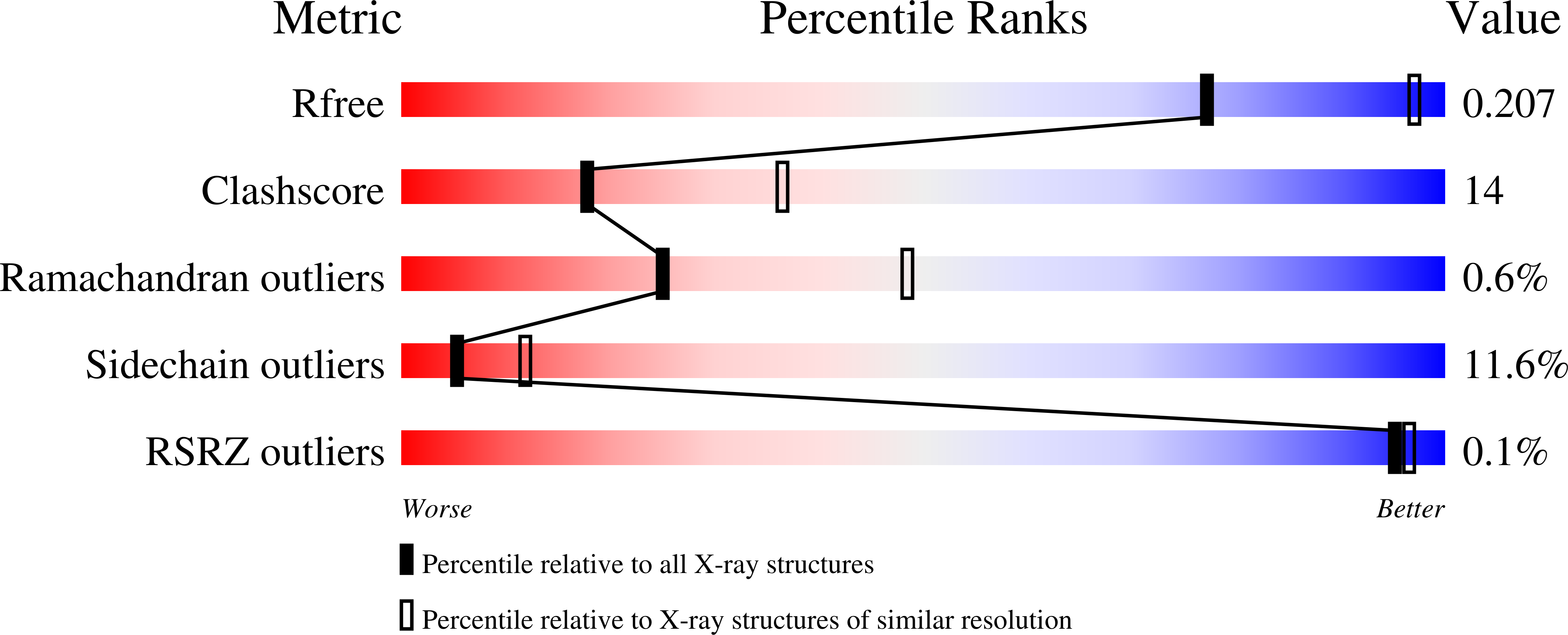
4A6G - PubMed Abstract:
Using directed evolution, a variant N-acetyl amino acid racemase (NAAAR G291D/F323Y) has been developed with up to 6-fold higher activity than the wild-type on a range of N-acetylated amino acids. The variant has been coupled with an enantiospecific acylase to give a preparative scale dynamic kinetic resolution which allows 98% conversion of N-acetyl-DL-allylglycine into D-allylglycine in 18 h at high substrate concentrations (50 g L(-1)). This is the first example of NAAAR operating under conditions which would allow it to be successfully used on an industrial scale for the production of enantiomerically pure ¦Á-amino acids. X-ray crystal analysis of the improved NAAAR variant allowed a comparison with the wild-type enzyme. We postulate that a network of novel interactions that result from the introduction of the two side chains is the source of improved catalytic performance.
Organizational Affiliation:
The EastChem School of Chemistry, Joseph Black Building, The University of Edinburgh, Edinburgh, EH9 3JJ, UK.