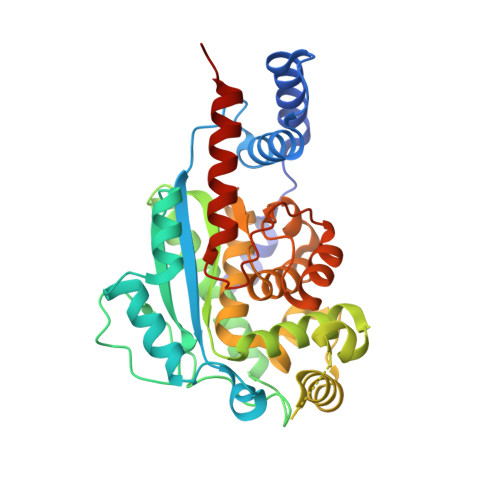
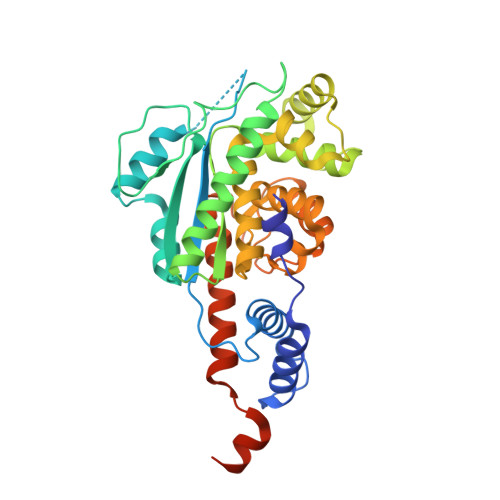
Structural Insights into the Function of the Nicotinate Mononucleotide:phenol/p-cresol Phosphoribosyltransferase (ArsAB) Enzyme from Sporomusa ovata.
Newmister, S.A., Chan, C.H., Escalante-Semerena, J.C., Rayment, I.(2012) Biochemistry 51: 8571-8582
- PubMed: 23039029
- DOI: https://doi.org/10.1021/bi301142h
- Primary Citation of Related Structures:
4HDK, 4HDM, 4HDN, 4HDR, 4HDS - PubMed Abstract:
Cobamides (Cbas) are cobalt (Co) containing tetrapyrrole-derivatives involved in enzyme-catalyzed carbon skeleton rearrangements, methyl-group transfers, and reductive dehalogenation. The biosynthesis of cobamides is complex and is only performed by some bacteria and achaea. Cobamides have an upper (Co¦Â) ligand (5'-deoxyadenosyl or methyl) and a lower (Co¦Á) ligand base that contribute to the axial Co coordinations. The identity of the lower Co¦Á ligand varies depending on the organism synthesizing the Cbas. The homoacetogenic bacterium Sporomusa ovata synthesizes two unique phenolic cobamides (i.e., Co¦Á-(phenolyl/p-cresolyl)cobamide), which are used in the catabolism of methanol and 3,4-dimethoxybenzoate by this bacterium. The S. ovata ArsAB enzyme activates a phenolic lower ligand prior to its incorporation into the cobamide. ArsAB consists of two subunits, both of which are homologous (¡«35% identity) to the well-characterized Salmonella enterica CobT enzyme, which transfers nitrogenous bases such as 5,6-dimethylbenzimidazole (DMB) and adenine, but cannot utilize phenolics. Here we report the three-dimensional structure of ArsAB, which shows that the enzyme forms a pseudosymmetric heterodimer, provide evidence that only the ArsA subunit has base:phosphoribosyl-transferase activity, and propose a mechanism by which phenolic transfer is facilitated by an activated water molecule.
Organizational Affiliation:
Departments of Biochemistry, University of Wisconsin, Madison, WI 53706, USA.