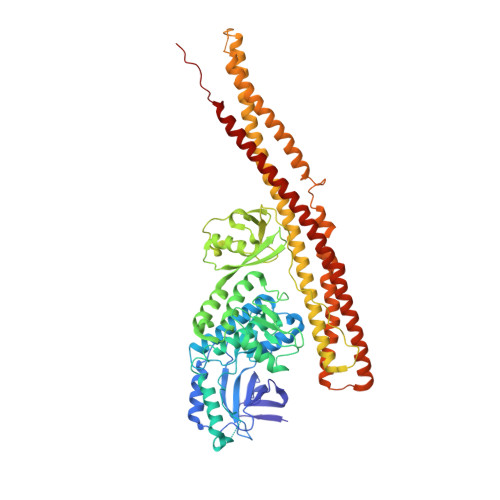
Structural Insights into the Functions of TBK1 in Innate Antimicrobial Immunity.
Shu, C., Sankaran, B., Chaton, C.T., Herr, A.B., Mishra, A., Peng, J., Li, P.(2013) Structure 21: 1137-1148
- PubMed: 23746807
- DOI: https://doi.org/10.1016/j.str.2013.04.025
- Primary Citation of Related Structures:
4JL9, 4JLC - PubMed Abstract:
Tank-binding kinase 1 (TBK1) is a serine/threonine protein-kinase mediating innate antimicrobial immunity. TBK1 is involved in the signaling of TLRs, RLRs, and STING-mediated sensing of cytosolic DNA. Stimulation of these receptors results in the activation of TBK1, which phosphorylates interferon regulatory factor (IRF)-3. Phosphorylated IRF-3 translocates into the nucleus to initiate the transcription of the interferon (IFN)-¦Â gene. Here, we show that TBK1 is activated by autophosphorylation at residue Ser172. Structures of TBK1 bound to two inhibitors showed that TBK1 has the I¦ÊB kinase fold with three distinct domains: the kinase domain, the ubiquitin-like domain, and the scaffold and dimerization domain. However, the overall structures of the TBK1 monomer and its dimer are different from IKK¦Â in the arrangements of the three domains and in dimer formation. Phosphorylation of IRF-3 by TBK1 in?vitro results in its oligomerization, and phosphorylation of residue Ser386 plays a key role in IRF-3 activation.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Texas A&M University, College Station, TX 77843-2128, USA.