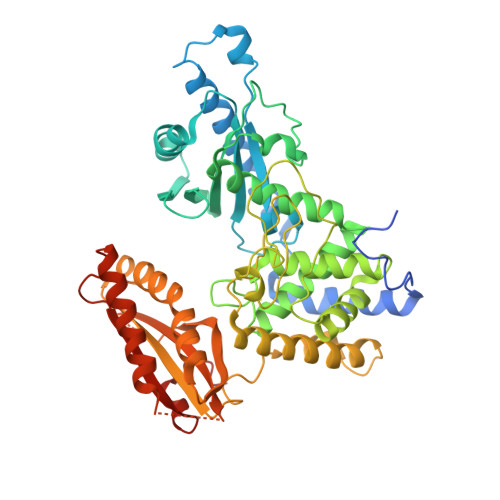
Crystal structure of human poly(a) polymerase gamma reveals a conserved catalytic core for canonical poly(a) polymerases.
Yang, Q., Nausch, L.W., Martin, G., Keller, W., Doublie, S.(2014) J Mol Biol 426: 43-50
- PubMed: 24076191
- DOI: https://doi.org/10.1016/j.jmb.2013.09.025
- Primary Citation of Related Structures:
4LT6 - PubMed Abstract:
In eukaryotes, the poly(A) tail added at the 3' end of an mRNA precursor is essential for the regulation of mRNA stability and the initiation of translation. Poly(A) polymerase (PAP) is the enzyme that catalyzes the poly(A) addition reaction. Multiple isoforms of PAP have been identified in vertebrates, which originate from gene duplication, alternative splicing or post-translational modifications. The complexity of PAP isoforms suggests that they might play different roles in the cell. Phylogenetic studies indicate that vertebrate PAPs are grouped into three clades termed ¦Á, ¦Â and ¦Ã, which originated from two gene duplication events. To date, all the available PAP structures are from the PAP¦Á clade. Here, we present the crystal structure of the first representative of the PAP¦Ã clade, human PAP¦Ã bound to cordycepin triphosphate (3'dATP) and Ca(2+). The structure revealed that PAP¦Ã closely resembles its PAP¦Á ortholog. An analysis of residue conservation reveals a conserved catalytic binding pocket, whereas residues at the surface of the polymerase are more divergent.
Organizational Affiliation:
Department of Microbiology and Molecular Genetics, University of Vermont, Stafford Hall, 95 Carrigan Drive, Burlington, VT 05405-0068, USA. Electronic address: yang@crystal.harvard.edu.