Molecular Mechanism of Avibactam-Mediated beta-Lactamase Inhibition.
King, D.T., King, A.M., Lal, S.M., Wright, G.D., Strynadka, N.C.(2015) ACS Infect Dis 1: 175-184
- PubMed: 27622530
- DOI: https://doi.org/10.1021/acsinfecdis.5b00007
- Primary Citation of Related Structures:
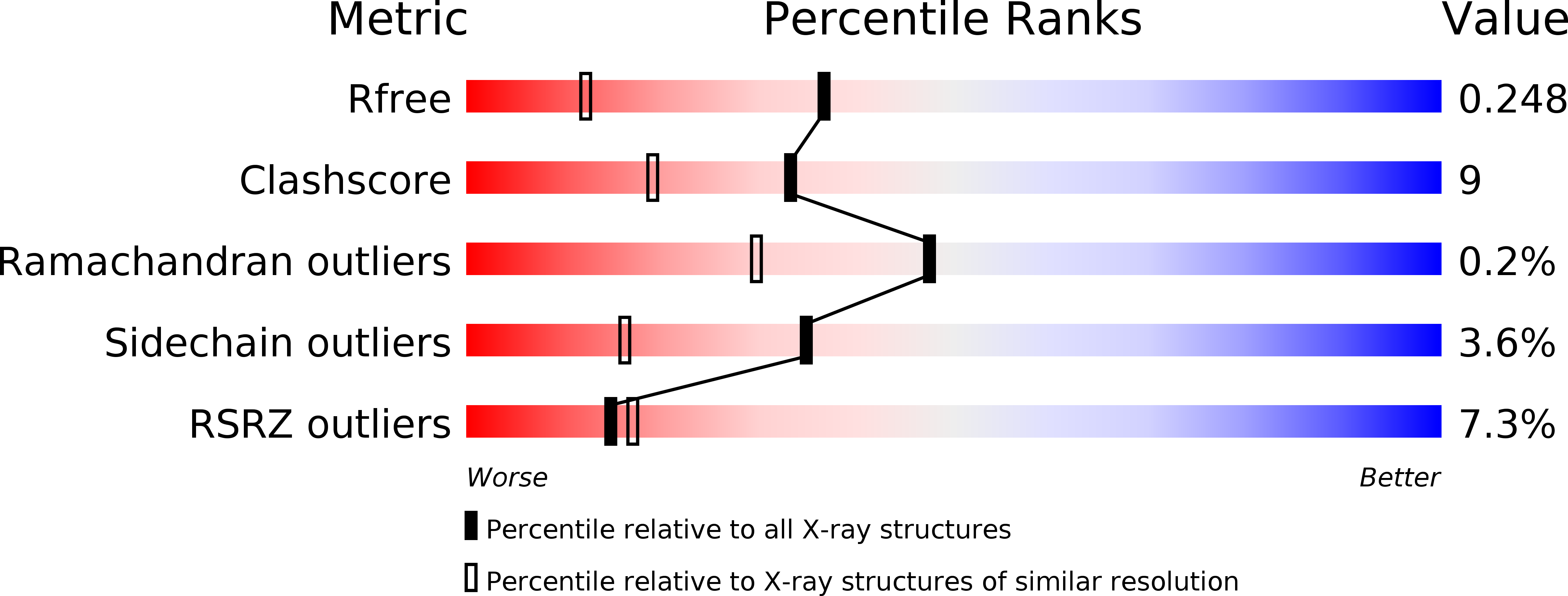
4S2I, 4S2J, 4S2K, 4S2N, 4S2O, 4S2P - PubMed Abstract:
Emerging ¦Â-lactamase-mediated resistance is threatening the clinical utility of the single most prominent class of antibacterial agents used in medicine, the ¦Â-lactams. The diazabicyclooctane avibactam is able to inhibit a wider range of serine ¦Â-lactamases than has been previously observed with ¦Â-lactamase inhibitors such as the widely prescribed clavulanic acid. However, despite its broad-spectrum activity, variable levels of inhibition have been observed for molecular class D ¦Â-lactamases. In order to better understand the molecular basis and spectrum of inhibition by avibactam, we provide structural and mechanistic analysis of the compound in complex with important class A and D serine ¦Â-lactamases. Herein, we reveal the 1.7- and 2.0-?-resolution crystal structures of avibactam covalently bound to class D ¦Â-lactamases OXA-10 and OXA-48. Furthermore, a kinetic analysis of key active-site mutants for class A ¦Â-lactamase CTX-M-15 allows us to propose a validated mechanism for avibactam-mediated ¦Â-lactamase inhibition including a unique role for S130, which acts as a general base. This study provides molecular insights that will aid in the design and development of avibactam-based chemotherapeutic agents effective against emerging drug-resistant microorganisms.
Organizational Affiliation:
The Department of Biochemistry and Molecular Biology and Center for Blood Research, University of British Columbia , 2350 Health Sciences Mall, Vancouver, British Columbia V6T 1Z3, Canada.