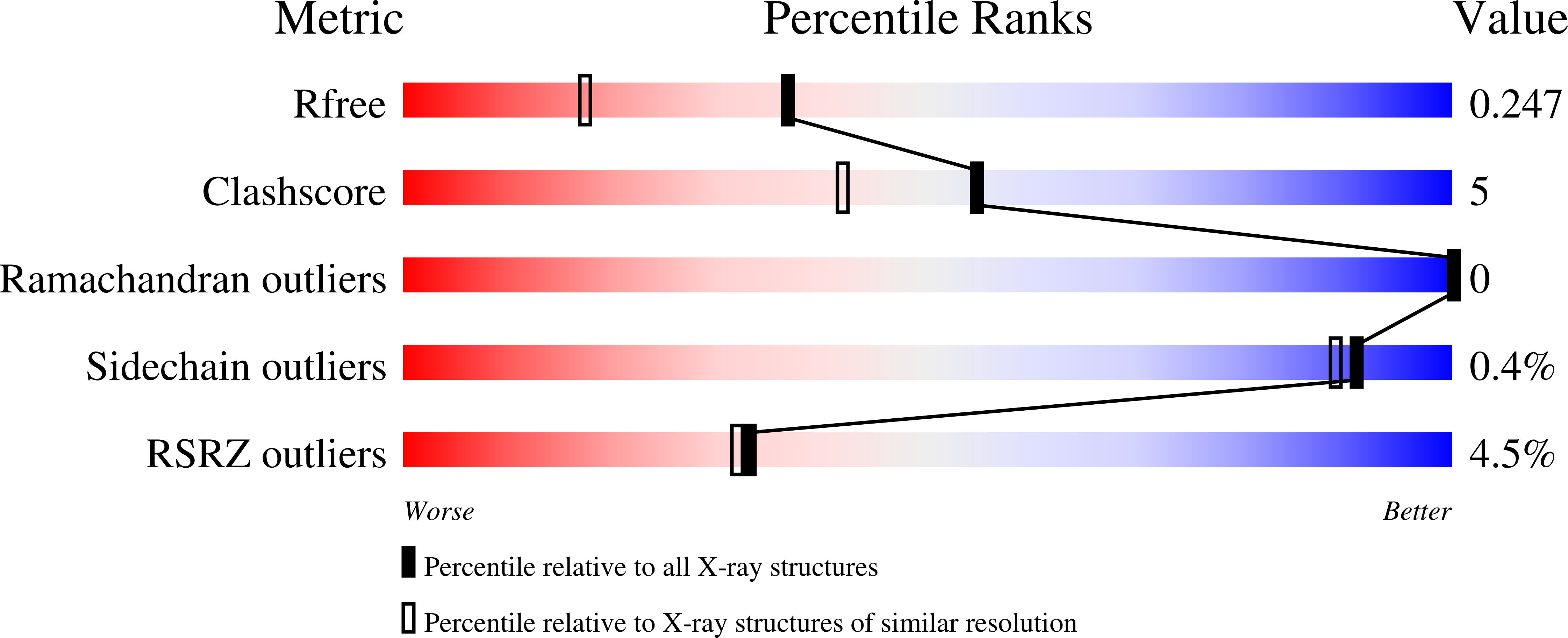
Crystal Structures of the Global Regulator DasR from Streptomyces coelicolor: Implications for the Allosteric Regulation of GntR/HutC Repressors.
Fillenberg, S.B., Friess, M.D., Korner, S., Bockmann, R.A., Muller, Y.A.(2016) PLoS One 11: e0157691-e0157691
- PubMed: 27337024
- DOI: https://doi.org/10.1371/journal.pone.0157691
- Primary Citation of Related Structures:
4ZS8, 4ZSB, 4ZSI, 4ZSK - PubMed Abstract:
Small molecule effectors regulate gene transcription in bacteria by altering the DNA-binding affinities of specific repressor proteins. Although the GntR proteins represent a large family of bacterial repressors, only little is known about the allosteric mechanism that enables their function. DasR from Streptomyces coelicolor belongs to the GntR/HutC subfamily and specifically recognises operators termed DasR-responsive elements (dre-sites). Its DNA-binding properties are modulated by phosphorylated sugars. Here, we present several crystal structures of DasR, namely of dimeric full-length DasR in the absence of any effector and of only the effector-binding domain (EBD) of DasR without effector or in complex with glucosamine-6-phosphate (GlcN-6-P) and N-acetylglucosamine-6-phosphate (GlcNAc-6-P). Together with molecular dynamics (MD) simulations and a comparison with other GntR/HutC family members these data allowed for a structural characterisation of the different functional states of DasR. Allostery in DasR and possibly in many other GntR/HutC family members is best described by a conformational selection model. In ligand-free DasR, an increased flexibility in the EBDs enables the attached DNA-binding domains (DBD) to sample a variety of different orientations and among these also a DNA-binding competent conformation. Effector binding to the EBDs of DasR significantly reorganises the atomic structure of the latter. However, rather than locking the orientation of the DBDs, the effector-induced formation of ¦Â-strand ¦Â* in the DBD-EBD-linker segment merely appears to take the DBDs 'on a shorter leash' thereby impeding the 'downwards' positioning of the DBDs that is necessary for a concerted binding of two DBDs of DasR to operator DNA.
Organizational Affiliation:
Division of Biotechnology, Department of Biology, Friedrich-Alexander University Erlangen-Nuremberg, Henkestr. 91, D-91052 Erlangen, Germany.