Studies on Aryl-Substituted Phenylalanines: Synthesis, Activity, and Different Binding Modes at AMPA Receptors.
Szymanska, E., Frydenvang, K., Pickering, D.S., Krintel, C., Nielsen, B., Kooshki, A., Zachariassen, L.G., Olsen, L., Kastrup, J.S., Johansen, T.N.(2016) J Med Chem 59: 448-461
- PubMed: 26653877
- DOI: https://doi.org/10.1021/acs.jmedchem.5b01666
- Primary Citation of Related Structures:
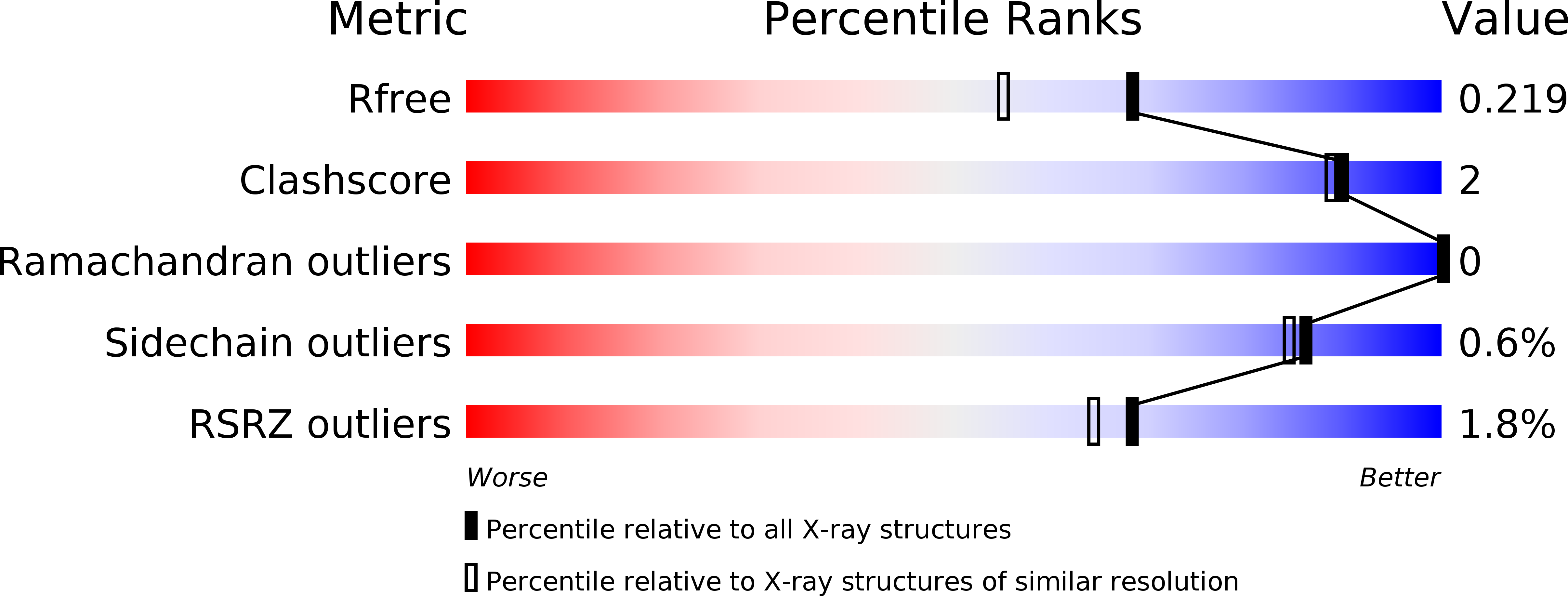
5CBR, 5CBS - PubMed Abstract:
A series of racemic aryl-substituted phenylalanines was synthesized and evaluated in vitro at recombinant rat GluA1-3, at GluK1-3, and at native AMPA receptors. The individual enantiomers of two target compounds, (RS)-2-amino-3-(3,4-dichloro-5-(5-hydroxypyridin-3-yl)phenyl)propanoic acid 37 and (RS)-2-amino-3-(3'-hydroxybiphenyl-3-yl)propanoic acid 38, were characterized. (S)-37 and (R)-38 were identified as the only biologically active isomers, both being antagonists at GluA2 receptors with Kb of 1.80 and 3.90 ¦̀M, respectively. To address this difference in enantiopharmacology, not previously seen for amino acid-based AMPA receptor antagonists, X-ray crystal structures of both eutomers in complex with the GluA2 ligand binding domain were solved. The cocrystal structures of (S)-37 and (R)-38 showed similar interactions of the amino acid parts but unexpected and different orientations and interactions of the biaromatic parts of the ligands inside the binding site, with (R)-38 having a binding mode not previously identified for amino acid-based antagonists.
Organizational Affiliation:
Department of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen , 2100 Copenhagen, Denmark.