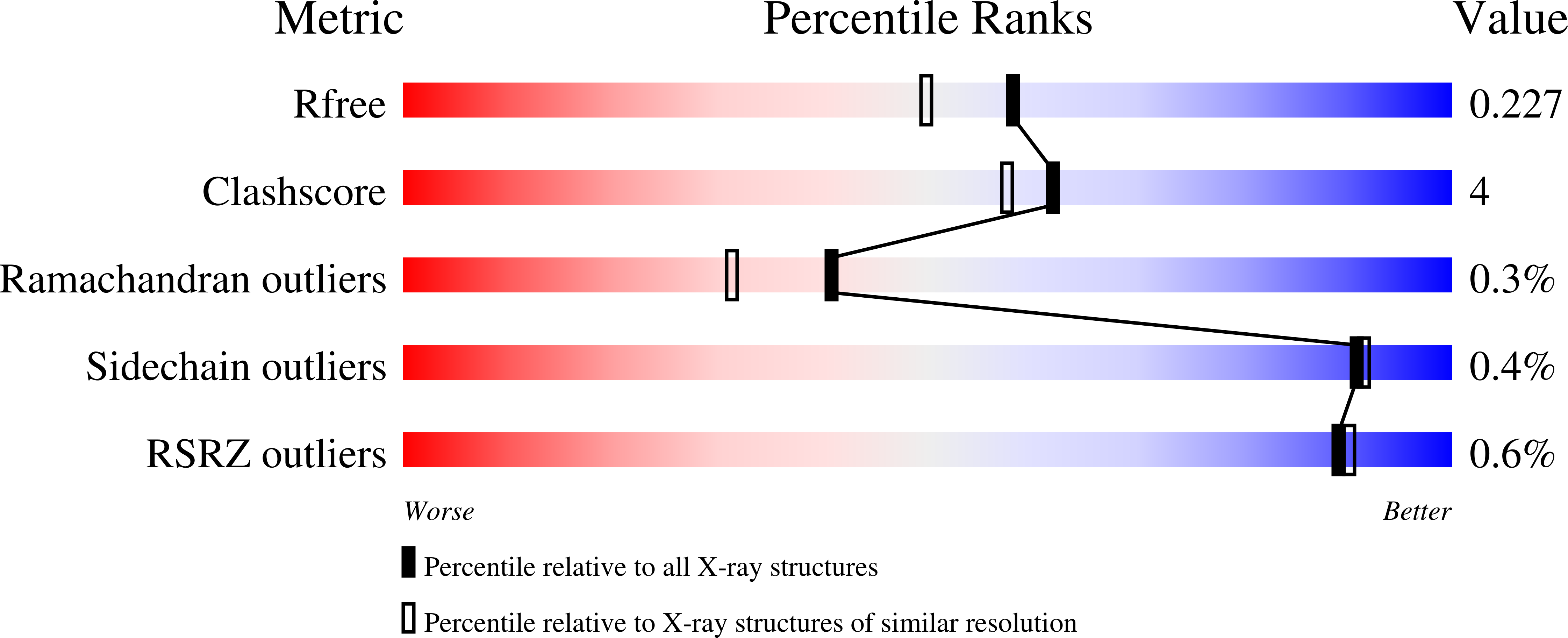
Structures of FOX-4 Cephamycinase in Complex with Transition-State Analog Inhibitors.
Lefurgy, S.T., Caselli, E., Taracila, M.A., Malashkevich, V.N., Biju, B., Papp-Wallace, K.M., Bonanno, J.B., Prati, F., Almo, S.C., Bonomo, R.A.(2020) Biomolecules 10
- PubMed: 32349291
- DOI: https://doi.org/10.3390/biom10050671
- Primary Citation of Related Structures:
5CHJ, 5CHM - PubMed Abstract:
Boronic acid transition-state analog inhibitors (BATSIs) are partners with ¦Â-lactam antibiotics for the treatment of complex bacterial infections. Herein, microbiological, biochemical, and structural findings on four BATSIs with the FOX-4 cephamycinase, a class C ¦Â-lactamase that rapidly hydrolyzes cefoxitin, are revealed. FOX-4 is an extended-spectrum class C cephalosporinase that demonstrates conformational flexibility when complexed with certain ligands. Like other ¦Â-lactamases of this class, studies on FOX-4 reveal important insights into structure-activity relationships. We show that SM23, a BATSI, shows both remarkable flexibility and affinity, binding similarly to other ¦Â-lactamases, yet retaining an IC 50 value < 0.1 ¦ÌM. Our analyses open up new opportunities for the design of novel transition-state analogs of class C enzymes.
Organizational Affiliation:
Department of Chemistry, Hofstra University, Hempstead, NY 11549, USA.