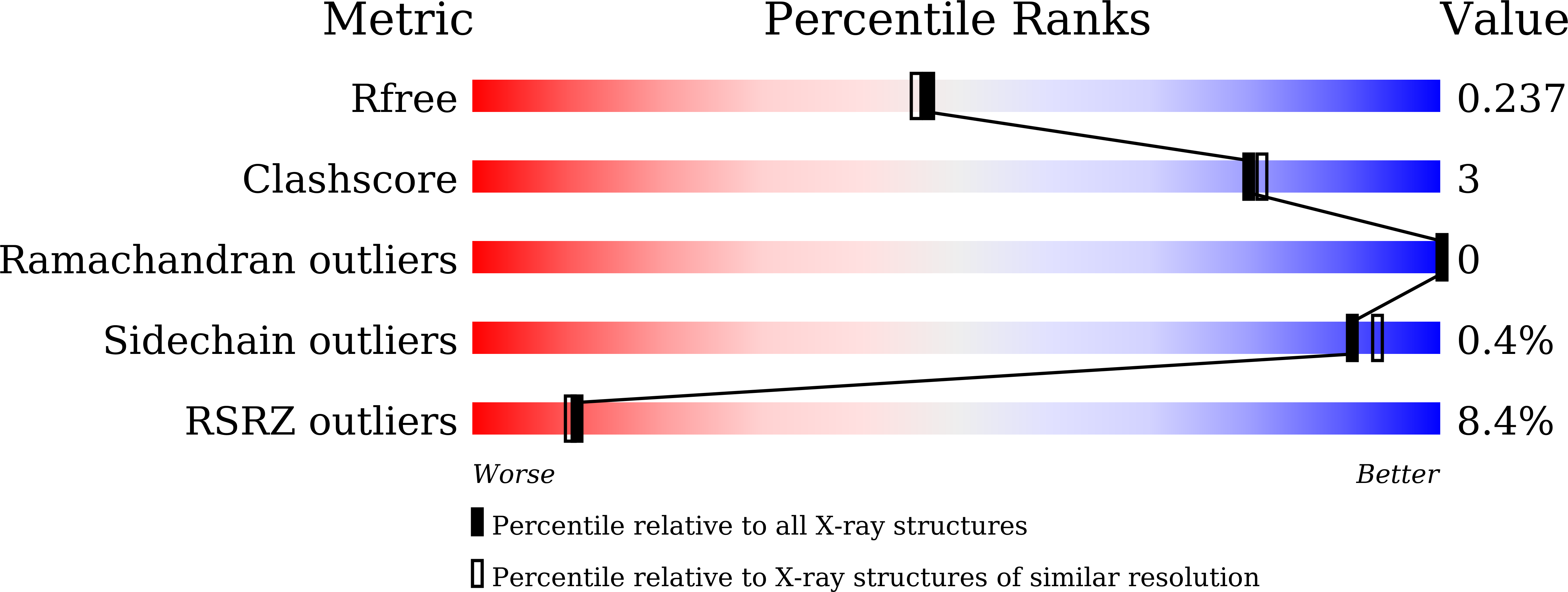
Structural Basis for the Enhanced Anti-Diabetic Efficacy of Lobeglitazone on PPAR gamma.
Jang, J.Y., Bae, H., Lee, Y.J., Choi, Y.I., Kim, H.J., Park, S.B., Suh, S.W., Kim, S.W., Han, B.W.(2018) Sci Rep 8: 31-31
- PubMed: 29311579
- DOI: https://doi.org/10.1038/s41598-017-18274-1
- Primary Citation of Related Structures:
5YCN, 5YCP - PubMed Abstract:
Peroxisome proliferator-activated receptor Ž├ (PPARŽ├) is a member of the nuclear receptor superfamily. It functions as a ligand-activated transcription factor and plays important roles in the regulation of adipocyte differentiation, insulin resistance, and inflammation. Here, we report the crystal structures of PPARŽ├ in complex with lobeglitazone, a novel PPARŽ├ agonist, and with rosiglitazone for comparison. The thiazolidinedione (TZD) moiety of lobeglitazone occupies the canonical ligand-binding pocket near the activation function-2 (AF-2) helix (i.e., helix H12) in ligand-binding domain as the TZD moiety of rosiglitazone does. However, the elongated p-methoxyphenol moiety of lobeglitazone interacts with the hydrophobic pocket near the alternate binding site of PPARŽ├. The extended interaction of lobeglitazone with the hydrophobic pocket enhances its binding affinity and could affect the cyclin-dependent kinase 5 (Cdk5)-mediated phosphorylation of PPARŽ├ at Ser245 (in PPARŽ├1 numbering; Ser273 in PPARŽ├2 numbering). Lobeglitazone inhibited the phosphorylation of PPARŽ├ at Ser245 in a dose-dependent manner and exhibited a better inhibitory effect on Ser245 phosphorylation than rosiglitazone did. Our study provides new structural insights into the PPARŽ├ regulation by TZD drugs and could be useful for the discovery of new PPARŽ├ ligands as an anti-diabetic drug, minimizing known side effects.
Organizational Affiliation:
Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul, 08826, Republic of Korea.