SAR studies on truxillic acid mono esters as a new class of antinociceptive agents targeting fatty acid binding proteins.
Yan, S., Elmes, M.W., Tong, S., Hu, K., Awwa, M., Teng, G.Y.H., Jing, Y., Freitag, M., Gan, Q., Clement, T., Wei, L., Sweeney, J.M., Joseph, O.M., Che, J., Carbonetti, G.S., Wang, L., Bogdan, D.M., Falcone, J., Smietalo, N., Zhou, Y., Ralph, B., Hsu, H.C., Li, H., Rizzo, R.C., Deutsch, D.G., Kaczocha, M., Ojima, I.(2018) Eur J Med Chem 154: 233-252
- PubMed: 29803996
- DOI: https://doi.org/10.1016/j.ejmech.2018.04.050
- Primary Citation of Related Structures:
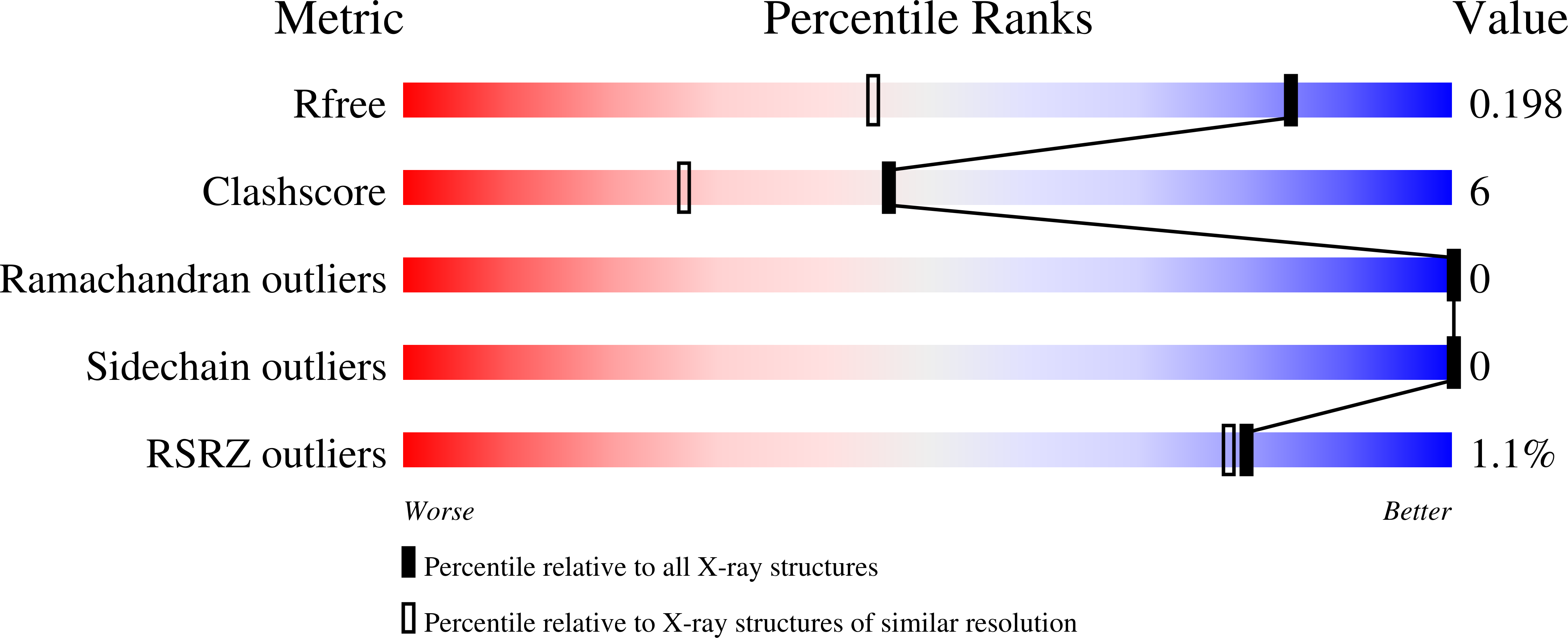
6AQ1 - PubMed Abstract:
Fatty acid binding proteins (FABPs) serve as critical modulators of endocannabinoid signaling by facilitating the intracellular transport of anandamide and whose inhibition potentiates anandamide signaling. Our previous work has identified a novel small-molecule FABP inhibitor, ¦Á-truxillic acid 1-naphthyl monoester (SB-FI-26, 3) that has shown efficacy as an antinociceptive and anti-inflammatory agent in rodent models. In the present work, we have performed an extensive SAR study on a series of 3-analogs as novel FABP inhibitors based on computer-aided inhibitor drug design and docking analysis, chemical synthesis and biological evaluations. The prediction of binding affinity of these analogs to target FABP3, 5 and 7 isoforms was performed using the AutoDock 4.2 program, using the recently determined co-crystal structures of 3 with FABP5 and FABP7. The compounds with high docking scores were synthesized and evaluated for their activities using a fluorescence displacement assay against FABP3, 5 and 7. During lead optimization, compound 3l emerged as a promising compound with the Ki value of 0.21?¦ÌM for FABP 5, 4-fold more potent than 3 (Ki, 0.81?¦ÌM). Nine compounds exhibit similar or better binding affinity than 3, including compounds 4b (Ki, 0.55?¦ÌM) and 4e (Ki, 0.68?¦ÌM). Twelve compounds are selective for FABP5 and 7 with >10?¦ÌM Ki values for FABP3, indicating a safe profile to avoid potential cardiotoxicity concerns. Compounds 4f, 4j and 4k showed excellent selectivity for FABP5 and would serve as other new lead compounds. Compound 3a possessed high affinity and high selectivity for FABP7. Compounds with moderate to high affinity for FABP5 displayed antinociceptive effects in mice while compounds with low FABP5 affinity lacked in?vivo efficacy. In?vivo pain model studies in mice revealed that exceeding hydrophobicity significantly affects the efficacy. Thus, among the compounds with high affinity to FABP5 in?vitro, the compounds with moderate hydrophobicity were identified as promising new lead compounds for the next round of optimization, including compounds 4b and 4j. For select cases, computational analysis of the observed SAR, especially the selectivity of new inhibitors to particular FABP isoforms, by comparing docking poses, interaction map, and docking energy scores has provided useful insights.
Organizational Affiliation:
Department of Chemistry, Stony Brook University, Stony Brook, NY, 11794-3400, United states.