Fluorescent indomethacin-dansyl conjugates utilize the membrane-binding domain of cyclooxygenase-2 to block the opening to the active site.
Xu, S., Uddin, M.J., Banerjee, S., Duggan, K., Musee, J., Kiefer, J.R., Ghebreselasie, K., Rouzer, C.A., Marnett, L.J.(2019) J Biological Chem 294: 8690-8698
- PubMed: 31000626
- DOI: https://doi.org/10.1074/jbc.RA119.007405
- Primary Citation of Related Structures:
6BL3, 6BL4 - PubMed Abstract:
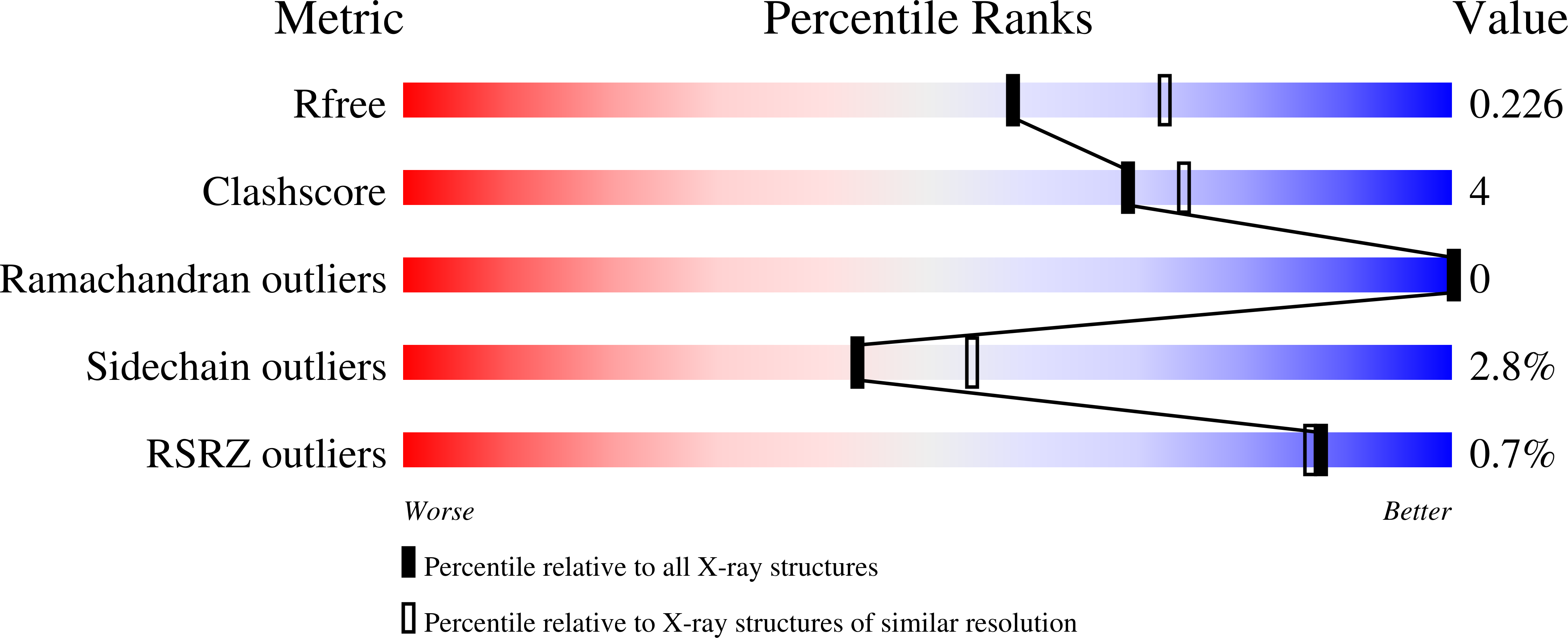
Many indomethacin amides and esters are cyclooxygenase-2 (COX-2)-selective inhibitors, providing a framework for the design of COX-2-targeted imaging and cancer chemotherapeutic agents. Although previous studies have suggested that the amide or ester moiety of these inhibitors binds in the lobby region, a spacious alcove within the enzyme's membrane-binding domain, structural details have been lacking. Here, we present observations on the crystal complexes of COX-2 with two indomethacin-dansyl conjugates (compounds 1 and 2) at 2.22-? resolution. Both compounds are COX-2-selective inhibitors with IC 50 values of 0.76 and 0.17 ¦̀m, respectively. Our results confirmed that the dansyl moiety is localized in and establishes hydrophobic interactions and several hydrogen bonds with the lobby of the membrane-binding domain. We noted that in both crystal structures, the linker tethering indomethacin to the dansyl moiety passes through the constriction at the mouth of the COX-2 active site, resulting in displacement and disorder of Arg-120, located at the opening to the active site. Both compounds exhibited higher inhibitory potency against a COX-2 R120A variant than against the WT enzyme. Inhibition kinetics of compound 2 were similar to those of the indomethacin parent compound against WT COX-2, and the R120A substitution reduced the time dependence of COX inhibition. These results provide a structural basis for the further design and optimization of conjugated COX reagents for imaging of malignant or inflammatory tissues containing high COX-2 levels.
Organizational Affiliation:
From the A. B. Hancock Jr. Memorial Laboratory for Cancer Research, Departments of Biochemistry, Chemistry, and Pharmacology, Vanderbilt Institute of Chemical Biology, Center in Molecular Toxicology, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, Tennessee 37232.