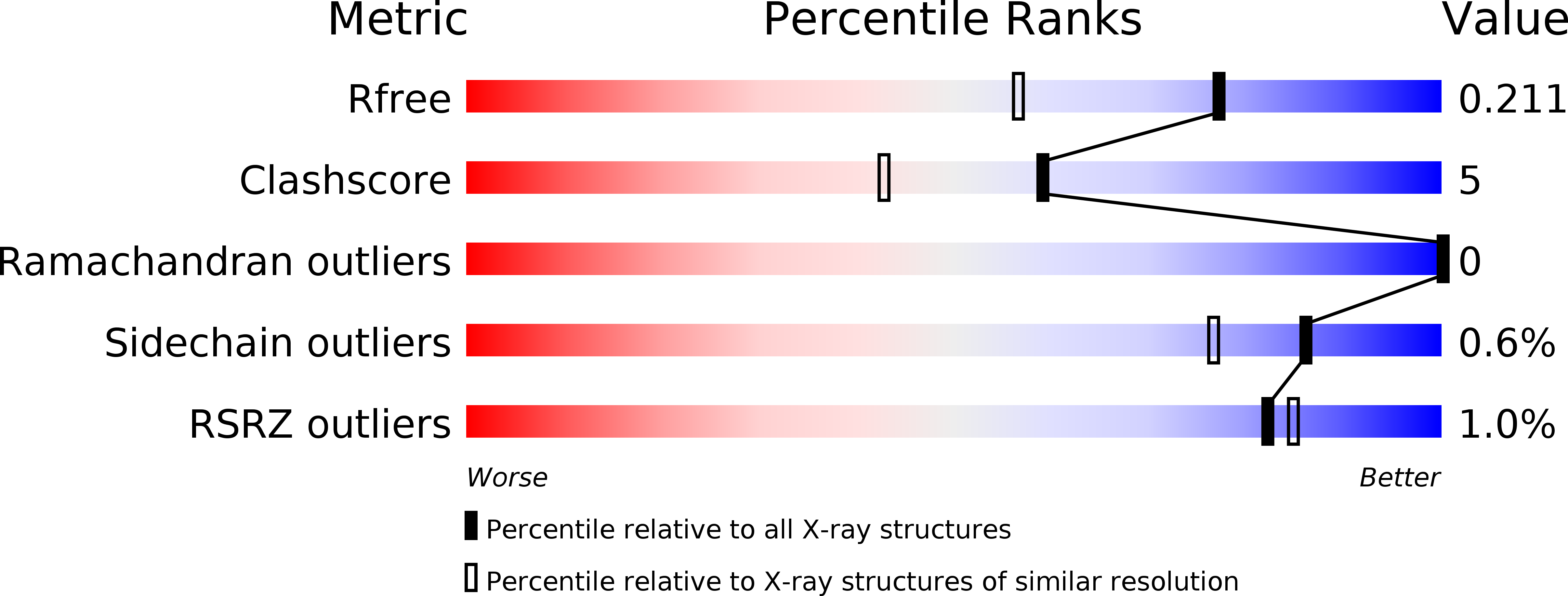
Crystal structure of the first eukaryotic bilin reductaseGtPEBB reveals a flipped binding mode of dihydrobiliverdin.
Sommerkamp, J.A., Frankenberg-Dinkel, N., Hofmann, E.(2019) J Biological Chem 294: 13889-13901
- PubMed: 31366727
- DOI: https://doi.org/10.1074/jbc.RA119.009306
- Primary Citation of Related Structures:
6QWQ, 6QX6 - PubMed Abstract:
Phycobilins are light-harvesting pigments of cyanobacteria, red algae, and cryptophytes. The biosynthesis of phycoerythrobilin (PEB) is catalyzed by the subsequent action of two ferredoxin-dependent bilin reductases (FDBRs). Although 15,16-dihydrobiliverdin (DHBV):ferredoxin oxidoreductase (PebA) catalyzes the two-electron reduction of biliverdin IX¦Á to 15,16-DHBV, PEB:ferredoxin oxidoreductase (PebB) reduces this intermediate further to PEB. Interestingly, marine viruses encode the FDBR PebS combining both activities within one enzyme. Although PebA and PebS share a canonical fold with similar substrate-binding pockets, the structural determinants for the stereo- and regiospecific modification of their tetrapyrrole substrates are incompletely understood, also because of the lack of a PebB structure. Here, we solved the X-ray crystal structures of both substrate-free and -bound PEBB from the cryptophyte Guillardia theta at 1.90 and 1.65 ?, respectively. The structures of PEBB exhibit the typical ¦Á/¦Â/¦Á-sandwich fold. Interestingly, the open-chain tetrapyrrole substrate DHBV is bound in an unexpected flipped orientation within the canonical FDBR active site. Biochemical analyses of the WT enzyme and active site variants identified two central aspartate residues Asp-99 and Asp-219 as essential for catalytic activity. In addition, the conserved Arg-215 plays a critical role in substrate specificity, binding orientation, and active site integrity. Because these critical residues are conserved within certain FDBRs displaying A-ring reduction activity, we propose that they present a conserved mechanism for this reaction. The flipped substrate-binding mode indicates that two-electron reducing FDBRs utilize the same primary site within the binding pocket and that substrate orientation is the determinant for A- or D-ring regiospecificity.
Organizational Affiliation:
Protein Crystallography, Faculty of Biology and Biotechnology, Ruhr University Bochum, 44801 Bochum, Germany.