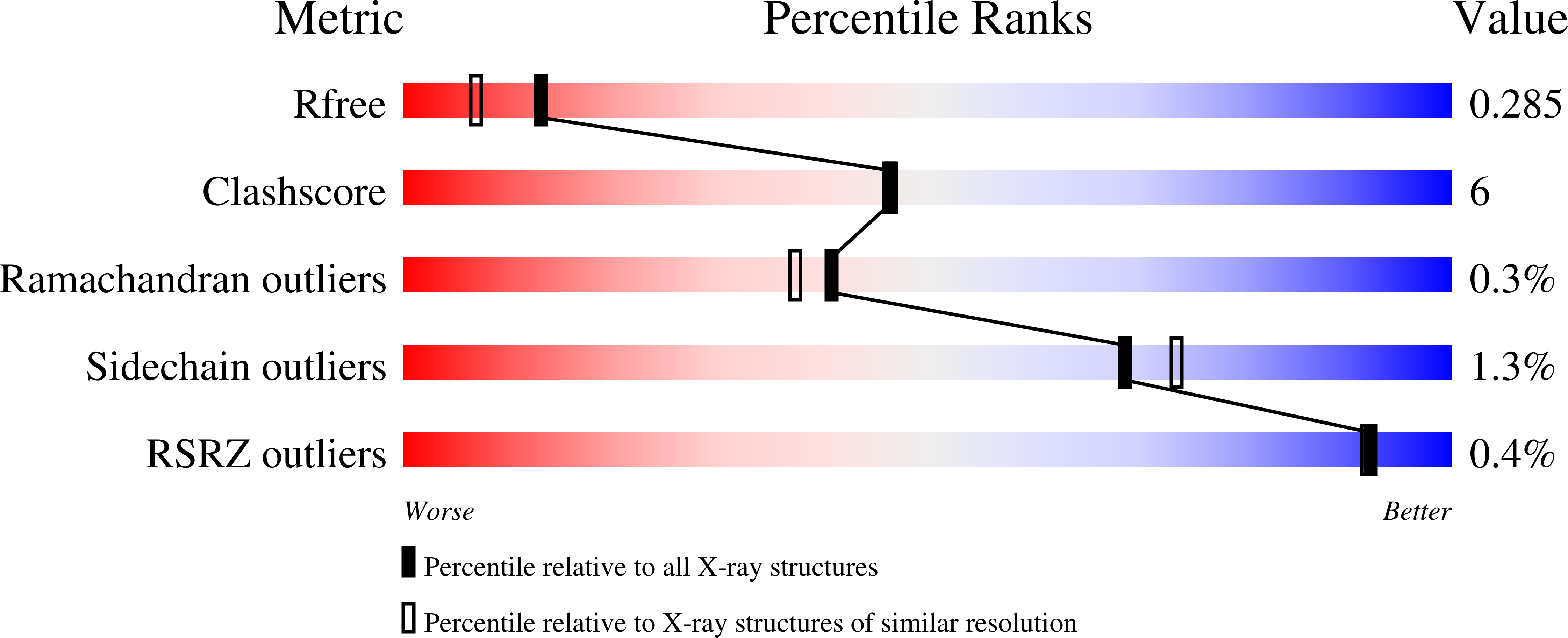
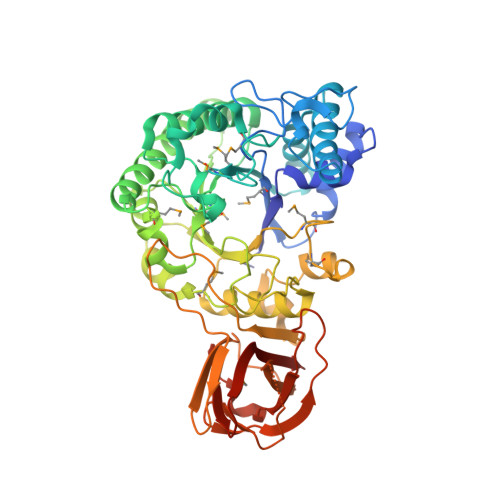
Inverting family GH156 sialidases define an unusual catalytic motif for glycosidase action.
Bule, P., Chuzel, L., Blagova, E., Wu, L., Gray, M.A., Henrissat, B., Rapp, E., Bertozzi, C.R., Taron, C.H., Davies, G.J.(2019) Nat Commun 10: 4816-4816
- PubMed: 31645552
- DOI: https://doi.org/10.1038/s41467-019-12684-7
- Primary Citation of Related Structures:
6RZD, 6S00, 6S04, 6S0E, 6S0F - PubMed Abstract:
Sialic acids are a family of related sugars that play essential roles in many biological events intimately linked to cellular recognition in both health and disease. Sialidases are therefore orchestrators of cellular biology and important therapeutic targets for viral infection. Here, we sought to define if uncharacterized sialidases would provide distinct paradigms in sialic acid biochemistry. We show that a recently discovered sialidase family, whose first member EnvSia156 was isolated from hot spring metagenomes, defines an unusual structural fold and active centre constellation, not previously described in sialidases. Consistent with an inverting mechanism, EnvSia156 reveals a His/Asp active center in which the His acts as a Br?nsted acid and Asp as a Br?nsted base in a single-displacement mechanism. A predominantly hydrophobic aglycone site facilitates accommodation of a variety of 2-linked sialosides; a versatility that offers the potential for glycan hydrolysis across a range of biological and technological platforms.
Organizational Affiliation:
Department of Chemistry, University of York, York, YO10 5DD, UK.