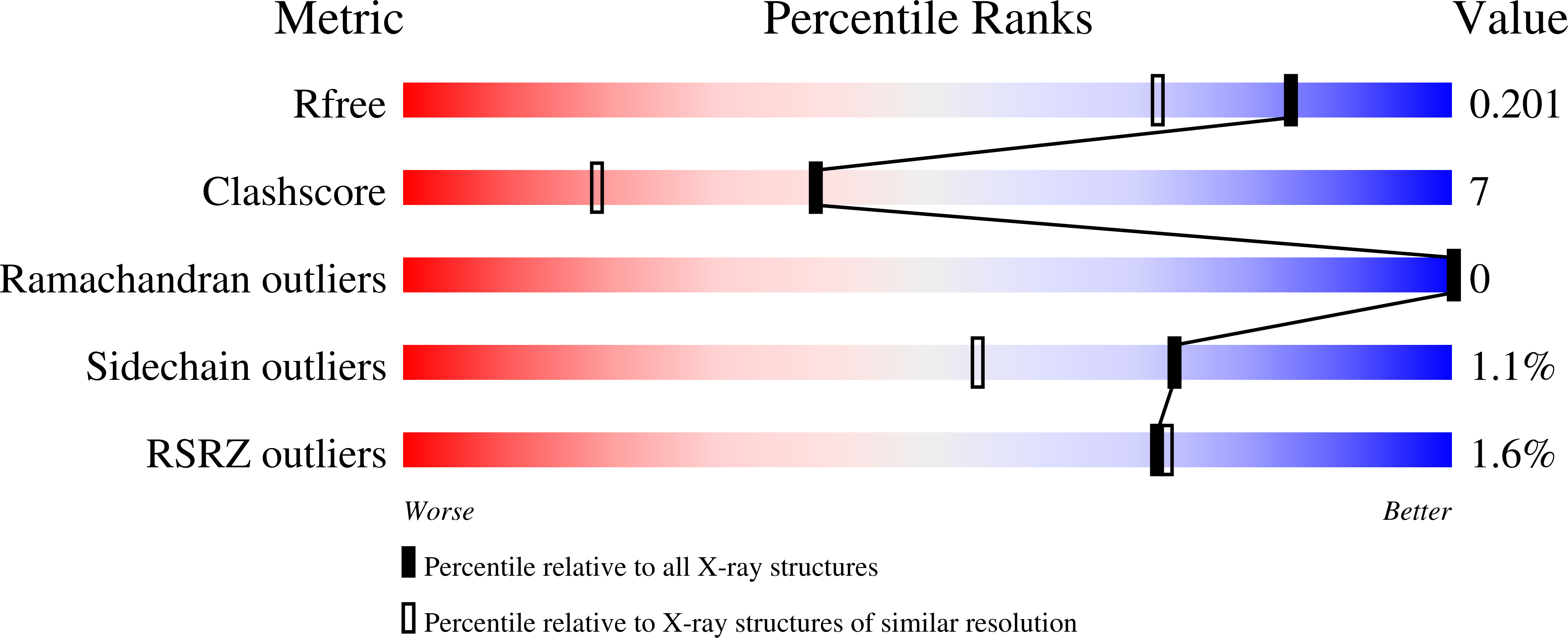
Crystal structures and calorimetry reveal catalytically relevant binding mode of coproporphyrin and coproheme in coproporphyrin ferrochelatase.
Hofbauer, S., Helm, J., Obinger, C., Djinovic-Carugo, K., Furtmuller, P.G.(2020) FEBS J 287: 2779-2796
- PubMed: 31794133
- DOI: https://doi.org/10.1111/febs.15164
- Primary Citation of Related Structures:
6RWV, 6SV3 - PubMed Abstract:
Coproporphyrin ferrochelatases (CpfCs, EC 4.99.1.9) insert ferrous iron into coproporphyrin III yielding coproheme. CpfCs are utilized by prokaryotic, mainly monoderm (Gram-positive) bacteria within the recently detected coproporphyrin-dependent (CPD) heme biosynthesis pathway. Here, we present a comprehensive study on CpfC from Listeria?monocytogenes (LmCpfC) including the first crystal structure of a coproheme-bound CpfC. Comparison of crystal structures of apo-LmCpfC and coproheme-LmCpfC allowed identification of structural rearrangements and of amino acids involved in tetrapyrrole macrocycle and Fe 2+ binding. Differential scanning calorimetry of apo-, coproporphyrin III-, and coproheme-LmCpfC underline the pronounced noncovalent interaction of both coproporphyrin and coproheme with the protein (¦¤T m ?=?11?ˇăC compared to apo-LmCpfC), which includes the propionates (p2, p4, p6, p7) and the amino acids Arg29, Arg45, Tyr46, Ser53, and Tyr124. Furthermore, the thermodynamics and kinetics of coproporphyrin III and coproheme binding to apo-LmCpfC is presented as well as the kinetics of insertion of ferrous iron into coproporphyrin III-LmCpfC that immediately leads to formation of ferric coproheme-LmCpfC (k cat /K M ?=?4.7?ˇÁ?10 5 ?m -1 ˇ¤s -1 ). We compare the crystal structure of coproheme-LmCpfC with available structures of CpfCs with artificial tetrapyrrole macrocycles and discuss our data on substrate binding, iron insertion and substrate release in the context of the CPD heme biosynthesis pathway. ENZYME: EC 4.99.1.9 DATABASE: pdb-codes of structural data in this work: 6RWV, 6SV3.
Organizational Affiliation:
Department of Chemistry, Institute of Biochemistry, BOKU - University of Natural Resources and Life Sciences, Vienna, Austria.