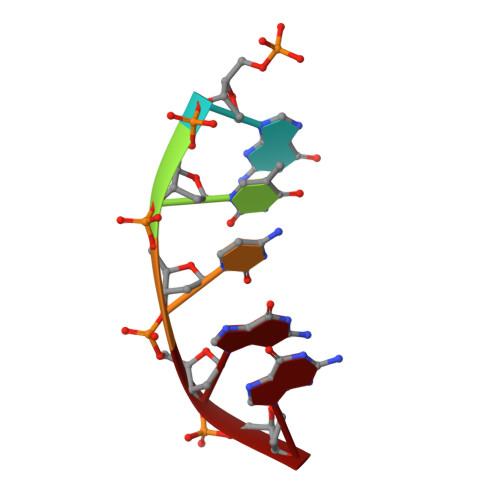
Molecular and structural characterization of oxidized ribonucleotide insertion into DNA by human DNA polymerase beta.
Smith, M.R., Alnajjar, K.S., Hoitsma, N.M., Sweasy, J.B., Freudenthal, B.D.(2020) J Biological Chem 295: 1613-1622
- PubMed: 31892517
- DOI: https://doi.org/10.1074/jbc.RA119.011569
- Primary Citation of Related Structures:
6UOK, 6UOL, 6UOM - PubMed Abstract:
During oxidative stress, inflammation, or environmental exposure, ribo- and deoxyribonucleotides are oxidatively modified. 8-Oxo-7,8-dihydro-2'-guanosine (8-oxo-G) is a common oxidized nucleobase whose deoxyribonucleotide form, 8-oxo-dGTP, has been widely studied and demonstrated to be a mutagenic substrate for DNA polymerases. Guanine ribonucleotides are analogously oxidized to r8-oxo-GTP, which can constitute up to 5% of the rGTP pool. Because ribonucleotides are commonly misinserted into DNA, and 8-oxo-G causes replication errors, we were motivated to investigate how the oxidized ribonucleotide is utilized by DNA polymerases. To do this, here we employed human DNA polymerase ¦Â (pol ¦Â) and characterized r8-oxo-GTP insertion with DNA substrates containing either a templating cytosine (nonmutagenic) or adenine (mutagenic). Our results show that pol ¦Â has a diminished catalytic efficiency for r8-oxo-GTP compared with canonical deoxyribonucleotides but that r8-oxo-GTP is inserted mutagenically at a rate similar to those of other common DNA replication errors ( i.e. ribonucleotide and mismatch insertions). Using FRET assays to monitor conformational changes of pol ¦Â with r8-oxo-GTP, we demonstrate impaired pol ¦Â closure that correlates with a reduced insertion efficiency. X-ray crystallographic analyses revealed that, similar to 8-oxo-dGTP, r8-oxo-GTP adopts an anti conformation opposite a templating cytosine and a syn conformation opposite adenine. However, unlike 8-oxo-dGTP, r8-oxo-GTP did not form a planar base pair with either templating base. These results suggest that r8-oxo-GTP is a potential mutagenic substrate for DNA polymerases and provide structural insights into how r8-oxo-GTP is processed by DNA polymerases.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Kansas Medical Center, Kansas City, Kansas 66160; Department of Cancer Biology, University of Kansas Medical Center, Kansas City, Kansas 66160.