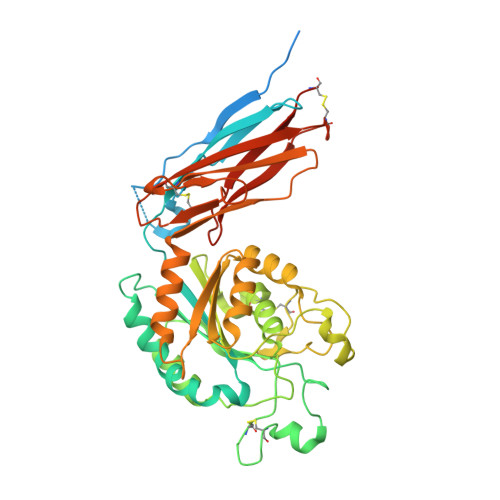
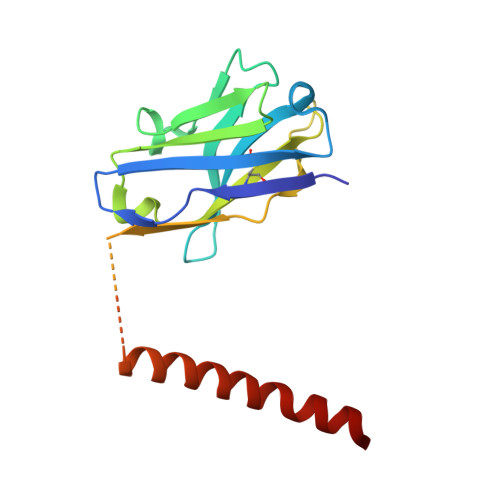
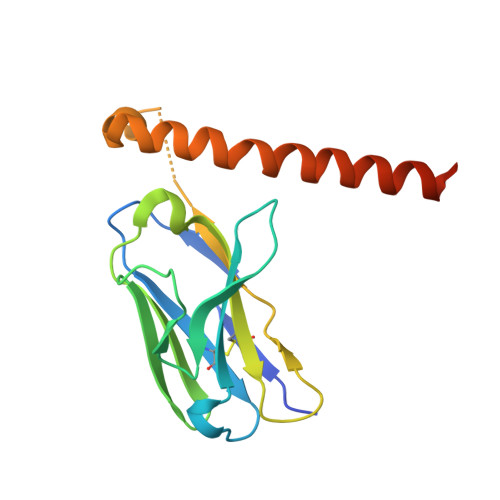
Structural mechanism of laminin recognition by integrin.
Arimori, T., Miyazaki, N., Mihara, E., Takizawa, M., Taniguchi, Y., Cabanas, C., Sekiguchi, K., Takagi, J.(2021) Nat Commun 12: 4012-4012
- PubMed: 34188035
- DOI: https://doi.org/10.1038/s41467-021-24184-8
- Primary Citation of Related Structures:
7CEA, 7CEB, 7CEC - PubMed Abstract:
Recognition of laminin by integrin receptors is central to the epithelial cell adhesion to basement membrane, but the structural background of this molecular interaction remained elusive. Here, we report the structures of the prototypic laminin receptor ¦Á6¦Â1 integrin alone and in complex with three-chain laminin-511 fragment determined via crystallography and cryo-electron microscopy, respectively. The laminin-integrin interface is made up of several binding sites located on all five subunits, with the laminin ¦Ă1 chain C-terminal portion providing focal interaction using two carboxylate anchor?points to bridge metal-ion dependent adhesion site of integrin ¦Â1 subunit and Asn189 of integrin ¦Á6 subunit. Laminin ¦Á5 chain also contributes to the affinity and specificity by making electrostatic interactions with large surface on the ¦Â-propeller domain of ¦Á6, part of which comprises an alternatively spliced X1 region. The propeller sheet corresponding to this region shows unusually high mobility, suggesting its unique role in ligand capture.
Organizational Affiliation:
Laboratory for Protein Synthesis and Expression, Institute for Protein Research, Osaka University, Suita, Osaka, Japan.