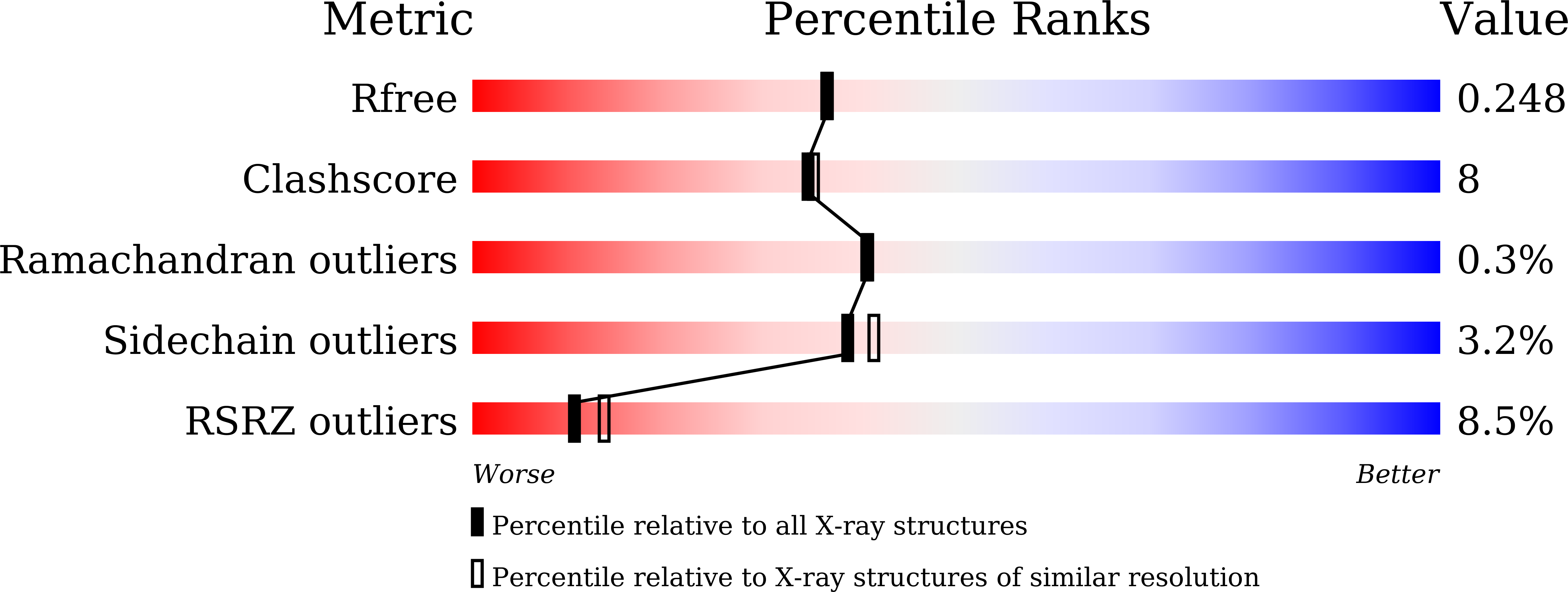
Structure of dye-decolorizing peroxidase from Bacillus subtilis in complex with veratryl alcohol.
Dhankhar, P., Dalal, V., Singh, V., Sharma, A.K., Kumar, P.(2021) Int J Biol Macromol 193: 601-608
- PubMed: 34687768
- DOI: https://doi.org/10.1016/j.ijbiomac.2021.10.100
- Primary Citation of Related Structures:
7DLK - PubMed Abstract:
Dye-decolorizing peroxidases (DyPs) are heme-containing peroxidases, which have promising application in biodegradation of phenolic lignin compounds and in detoxification of dyes. In this study, the crystal structure of BsDyP- veratryl alcohol (VA) complex delves deep into the binding of small substrate molecules within the DyP heme cavity. The biochemical analysis shows that BsDyP oxidizes the VA with a turnover number of 0.065?s -1 , followed by the oxidation of 2,6-dimethoxyphenol (DMP) and guaiacol with a comparable turnover number (k cat ) of 0.07?s -1 and 0.07?s -1 , respectively. Moreover, biophysical and computational studies reveal the comparable binding affinity of substrates to BsDyP and produce lower-energy stable BsDyP-ligand(s) complexes. All together with our previous findings, we are providing a complete structural description of substrate-binding sites in DyP. The structural insight of BsDyP helps to modulate its engineering to enhance the activity towards the oxidation of a wide range of substrates.
Organizational Affiliation:
Department of Biosciences and Bioengineering, Indian Institute of Technology Roorkee, 247667, India.