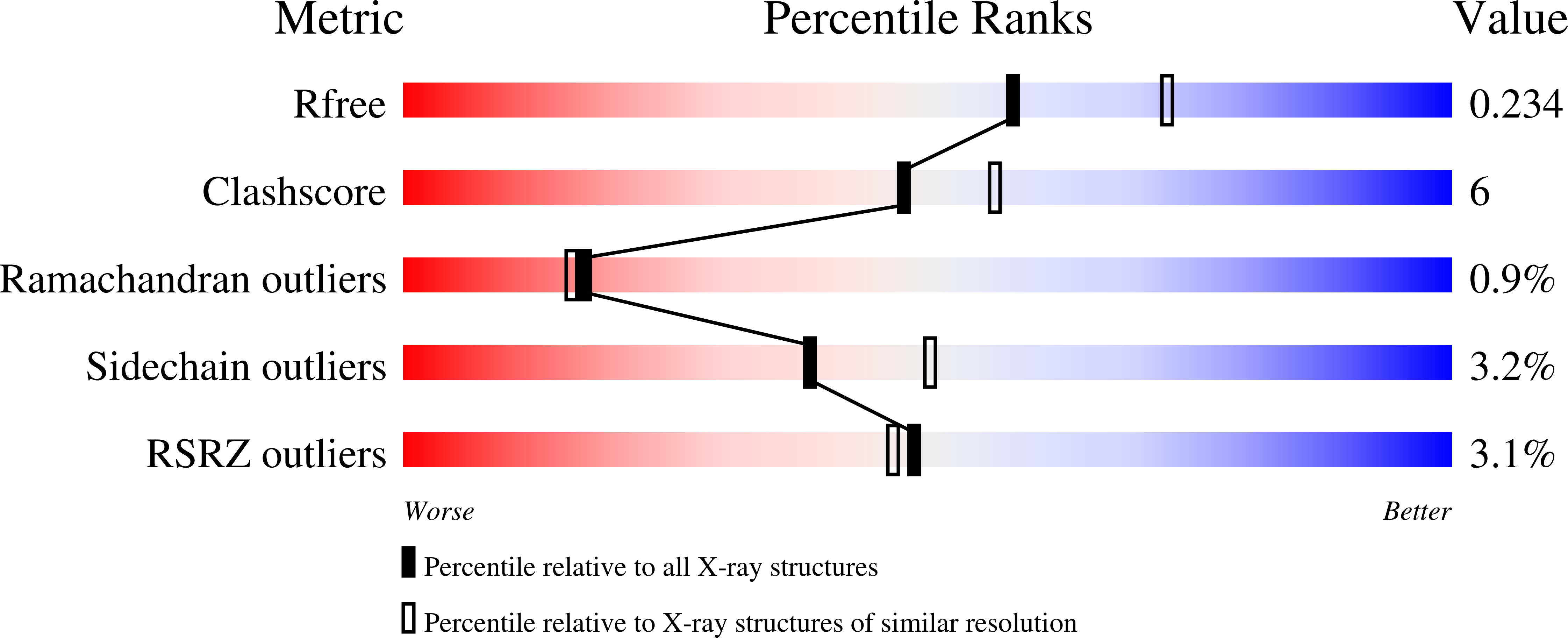
Cg10062 Catalysis Forges a Link between Acetylenecarboxylic Acid and Bacterial Metabolism.
Mathes Hewage, A., Nayebi Gavgani, H., Chi, D., Qiu, B., Geiger, J.H., Draths, K.(2021) Biochemistry 60: 3879-3886
- PubMed: 34910871
- DOI: https://doi.org/10.1021/acs.biochem.1c00524
- Primary Citation of Related Structures:
7MS0, 7MS1, 7MS3, 7MS8, 7MS9 - PubMed Abstract:
The reliance of biocatalysis on plant-derived carbon for the synthesis of fuels and chemicals places it in direct competition with food production for resources. A potential solution to this problem is development of a metabolic link between alternative carbon sources and bacterial metabolism. Acetylenecarboxylic acid, which can be synthesized from methane and carbon dioxide, could enable this connection. It was previously shown that the enzyme Cg10062 catalyzes hydration of acetylenecarboxylate to afford malonate semialdehyde. Subsequent hydration-dependent decarboxylation to form acetaldehyde (81%), which was also observed, limits its biocatalytic usefulness. Several Cg10062 variants including E114Q and E114D do not catalyze decarboxylation and provide malonate semialdehyde as the sole product, albeit with substantially reduced catalytic activity. To identify an efficient enzyme capable of catalyzing acetylenecarboxylate hydration without decarboxylation, we undertook a mechanistic investigation of Cg10062 using mutagenesis, kinetic characterization, and X-ray crystallography. Cg10062 is a member of the tautomerase superfamily of enzymes, characterized by their ¦Â-¦Á-¦Â protein fold and an N-terminal proline residue situated at the center of the enzyme active site. Along with Pro-1, five additional active site residues (His-28, Arg-70, Arg-73, Tyr-103, and Glu-114) are required for Cg10062 activity. Incubation of crystals of four catalytically slow variants of Cg10062 with acetylenecarboxylate resulted in atomic resolution structures of Pro-1 bound to a complete set of intermediates, fully elaborating the detailed mechanism of the enzyme and establishing the process to involve covalent catalysis. Further, the intermediate-bound E114D structure explains the mechanism governing decarboxylation suppression. Together, these studies provide the most detailed picture of the catalytic mechanism of a tautomerase enzyme to date.
Organizational Affiliation:
Department of Chemistry, Michigan State University, East Lansing, Michigan 48824, United States.