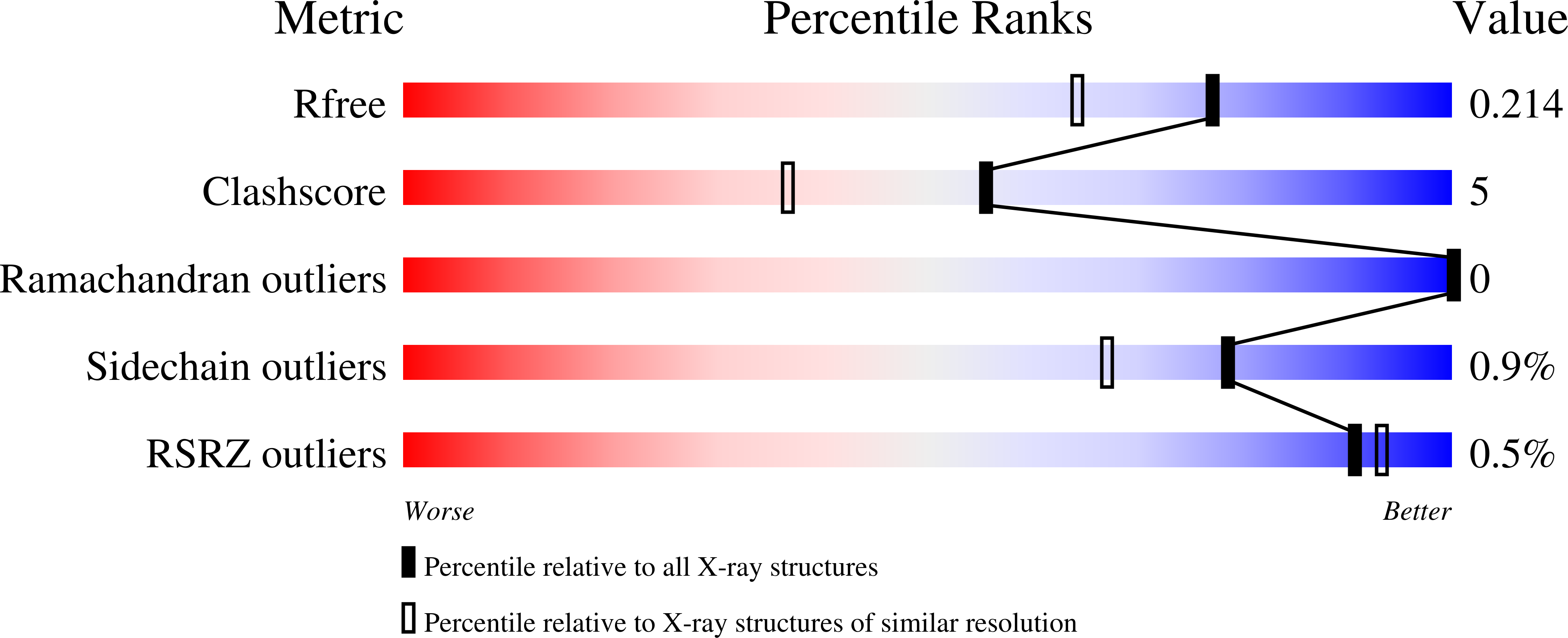
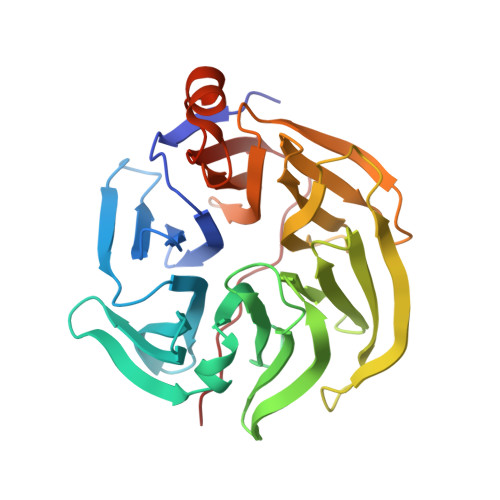
Three-dimensional structure of xylonolactonase from Caulobacter crescentus: A mononuclear iron enzyme of the 6-bladed beta-propeller hydrolase family.
Paakkonen, J., Hakulinen, N., Andberg, M., Koivula, A., Rouvinen, J.(2022) Protein Sci 31: 371-383
- PubMed: 34761460
- DOI: https://doi.org/10.1002/pro.4229
- Primary Citation of Related Structures:
7PLB, 7PLC, 7PLD - PubMed Abstract:
Xylonolactonase Cc XylC from Caulobacter crescentus catalyzes the hydrolysis of the intramolecular ester bond of d-xylonolactone. We have determined crystal structures of Cc XylC in complex with d-xylonolactone isomer analogues d-xylopyranose and (r)-(+)-4-hydroxy-2-pyrrolidinone at high resolution. Cc XylC has a 6-bladed ¦Â-propeller architecture, which contains a central open channel having the active site at one end. According to our previous native mass spectrometry studies, Cc XylC is able to specifically bind Fe 2+ . The crystal structures, presented here, revealed an active site bound metal ion with an octahedral binding geometry. The side chains of three amino acid residues, Glu18, Asn146, and Asp196, which participate in binding of metal ion are located in the same plane. The solved complex structures allowed suggesting a reaction mechanism for intramolecular ester bond hydrolysis in which the major contribution for catalysis arises from the carbonyl oxygen coordination of the xylonolactone substrate to the Fe 2+ . The structure of Cc XylC was compared with eight other ester hydrolases of the ¦Â-propeller hydrolase family. The previously published crystal structures of other ¦Â-propeller hydrolases contain either Ca 2+ , Mg 2+ , or Zn 2+ and show clear similarities in ligand and metal ion binding geometries to that of Cc XylC. It would be interesting to reinvestigate the metal binding specificity of these enzymes and clarify whether they are also able to use Fe 2+ as a catalytic metal. This could further expand our understanding of utilization of Fe 2+ not only in oxidative enzymes but also in hydrolases.
Organizational Affiliation:
Department of Chemistry, University of Eastern Finland, Joensuu, Finland.