Initial Steps to Engineer Coproheme Decarboxylase to Obtain Stereospecific Monovinyl, Monopropionyl Deuterohemes.
Michlits, H., Valente, N., Mlynek, G., Hofbauer, S.(2021) Front Bioeng Biotechnol 9: 807678-807678
- PubMed: 35141216
- DOI: https://doi.org/10.3389/fbioe.2021.807678
- Primary Citation of Related Structures:
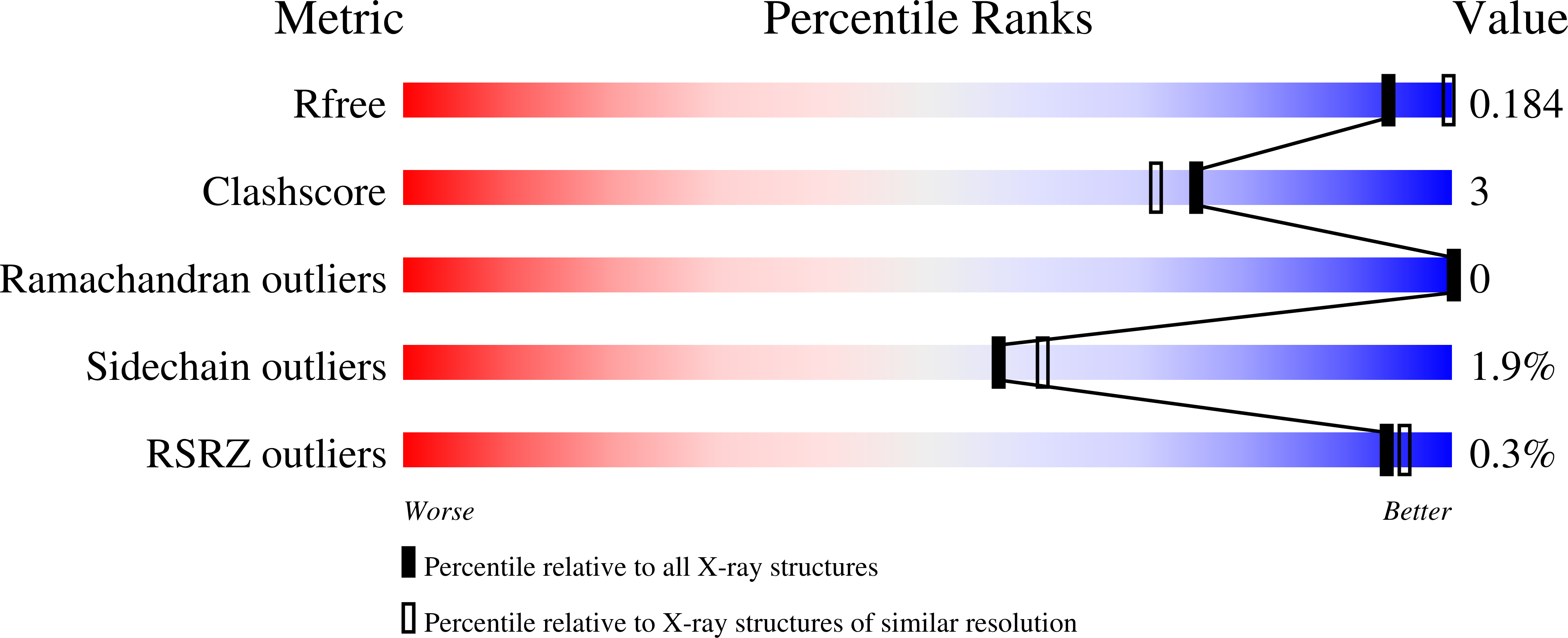
7Q4F, 7Q4G - PubMed Abstract:
The oxidative decarboxylation of coproheme to form heme b by coproheme decarboxylase is a stereospecific two-step reaction. In the first step, the propionate at position two (p2) is cleaved off the pyrrole ring A to form a vinyl group at this position. Subsequently, the propionate at position four (p4) on pyrrole ring B is cleaved off and heme b is formed. In this study, we attempted to engineer coproheme decarboxylase from Corynebacterium diphtheriae to alter the stereospecificity of this reaction. By introducing a tyrosine residue in proximity to the propionate at position 4, we were able to create a new radical center in the active site. However, the artificial Tyr183 ? radical could not be shown to catalyze any decarboxylation.
Organizational Affiliation:
Department of Chemistry, Institute of Biochemistry, University of Natural Resources and Life Sciences, Vienna, Austria.