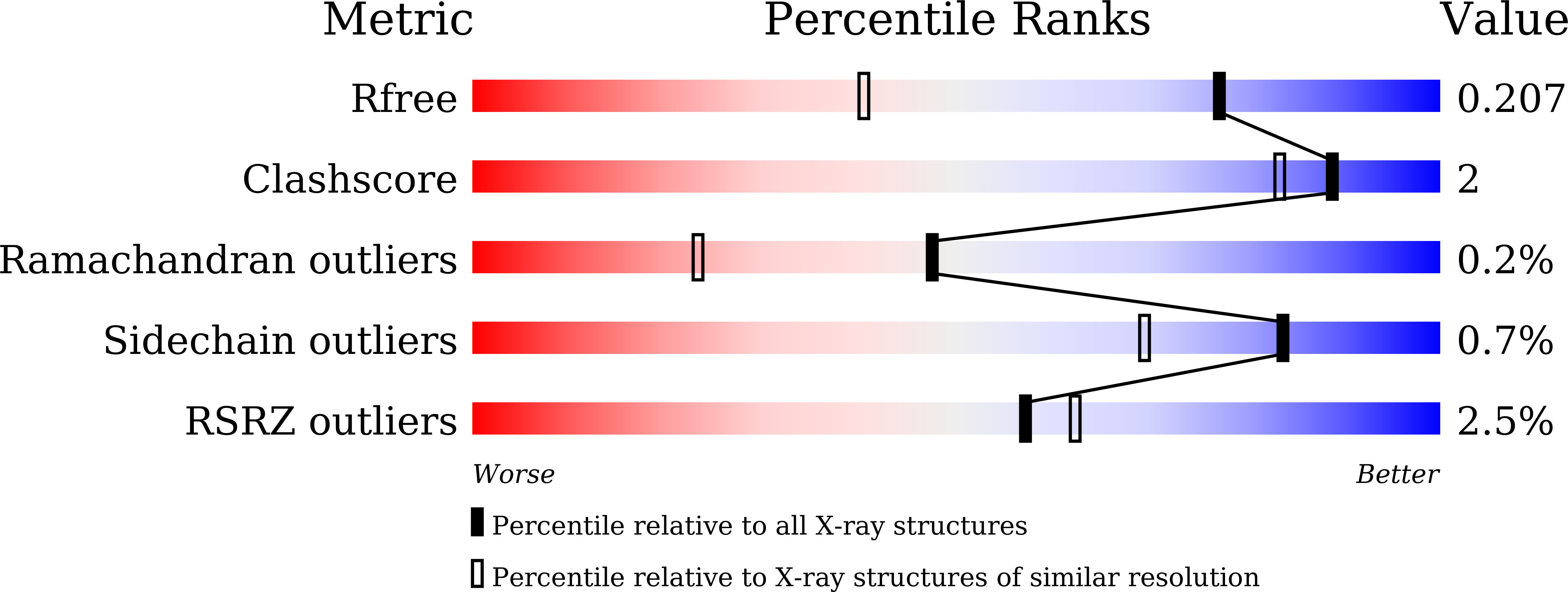
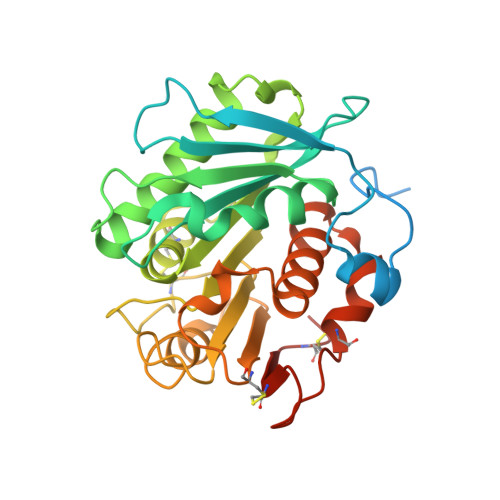
Discovery and rational engineering of PET hydrolase with both mesophilic and thermophilic PET hydrolase properties.
Hong, H., Ki, D., Seo, H., Park, J., Jang, J., Kim, K.J.(2023) Nat Commun 14: 4556-4556
- PubMed: 37507390
- DOI: https://doi.org/10.1038/s41467-023-40233-w
- Primary Citation of Related Structures:
7YM9, 7YME - PubMed Abstract:
Excessive polyethylene terephthalate (PET) waste causes a variety of problems. Extensive research focused on the development of superior PET hydrolases for PET biorecycling has been conducted. However, template enzymes employed in enzyme engineering mainly focused on IsPETase and leaf-branch compost cutinase, which exhibit mesophilic and thermophilic hydrolytic properties, respectively. Herein, we report a PET hydrolase from Cryptosporangium aurantiacum (CaPETase) that exhibits high thermostability and remarkable PET degradation activity at ambient temperatures. We uncover the crystal structure of CaPETase, which displays a distinct backbone conformation at the active site and residues forming the substrate binding cleft, compared with other PET hydrolases. We further develop a CaPETase M9 variant that exhibits robust thermostability with a T m of 83.2?ˇăC and 41.7-fold enhanced PET hydrolytic activity at 60?ˇăC compared with CaPETase WT . CaPETase M9 almost completely decompose both transparent and colored post-consumer PET powder at 55?ˇăC within half a day in a pH-stat bioreactor.
Organizational Affiliation:
School of Life Sciences, BK21 FOUR KNU Creative BioResearch Group, KNU Institute for Microorganisms, Kyungpook National University, Daegu, 41566, Republic of Korea.