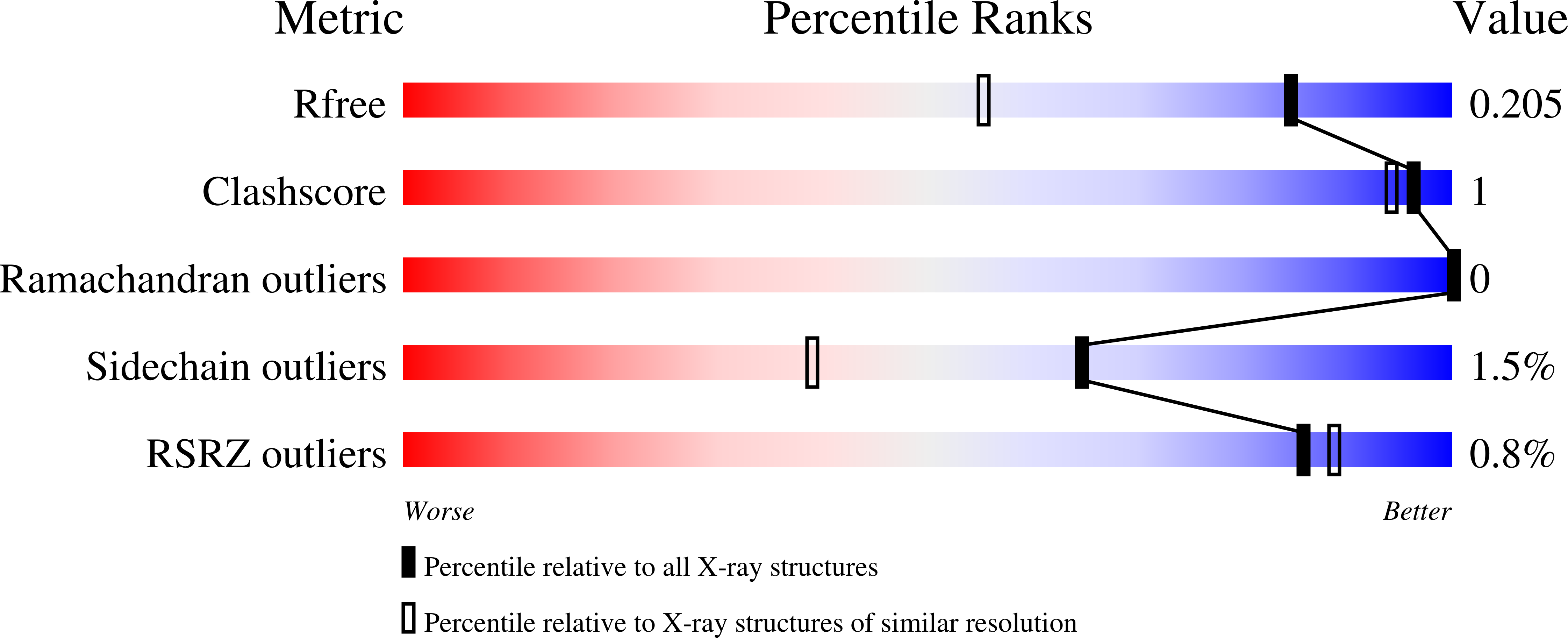
Interactions of hydrolyzed beta-lactams with the L1 metallo-beta-lactamase: Crystallography supports stereoselective binding of cephem/carbapenem products.
Hinchliffe, P., Calvopina, K., Rabe, P., Mojica, M.F., Schofield, C.J., Dmitrienko, G.I., Bonomo, R.A., Vila, A.J., Spencer, J.(2023) J Biol Chem 299: 104606-104606
- PubMed: 36924941
- DOI: https://doi.org/10.1016/j.jbc.2023.104606
- Primary Citation of Related Structures:
7ZO2, 7ZO3, 7ZO4, 7ZO5, 7ZO6, 7ZO7 - PubMed Abstract:
L1 is a dizinc subclass B3 metallo-¦Â-lactamase (MBL) that hydrolyzes most ¦Â-lactam antibiotics and is a key resistance determinant in the Gram-negative pathogen Stenotrophomonas maltophilia, an important cause of nosocomial infections in immunocompromised patients. L1 is not usefully inhibited by MBL inhibitors in clinical trials, underlying the need for further studies on L1 structure and mechanism. We describe kinetic studies and crystal structures of L1 in complex with hydrolyzed ¦Â-lactams from the penam (mecillinam), cephem (cefoxitin/cefmetazole), and carbapenem (tebipenem, doripenem, and panipenem) classes. Despite differences in their structures, all the ¦Â-lactam-derived products hydrogen bond to Tyr33, Ser221, and Ser225 and are stabilized by interactions with a conserved hydrophobic pocket. The carbapenem products were modeled as ¦¤ 1 -imines, with (2S)-stereochemistry. Their binding mode is determined by the presence of a 1¦Â-methyl substituent: the Zn-bridging hydroxide either interacts with the C-6 hydroxyethyl group (1¦Â-hydrogen-containing carbapenems) or is displaced by the C-6 carboxylate (1¦Â-methyl-containing carbapenems). Unexpectedly, the mecillinam product is a rearranged N-formyl amide rather than penicilloic acid, with the N-formyl oxygen interacting with the Zn-bridging hydroxide. NMR studies imply mecillinam rearrangement can occur nonenzymatically in solution. Cephem-derived imine products are bound with (3R)-stereochemistry and retain their 3' leaving groups, likely representing stable endpoints, rather than intermediates, in MBL-catalyzed hydrolysis. Our structures show preferential complex formation by carbapenem- and cephem-derived species protonated on the equivalent (¦Â) faces and so identify interactions that stabilize diverse hydrolyzed antibiotics. These results may be exploited in developing antibiotics, and ¦Â-lactamase inhibitors, that form long-lasting complexes with dizinc MBLs.
Organizational Affiliation:
School of Cellular and Molecular Medicine, University of Bristol, Biomedical Sciences Building, University Walk, Bristol, United Kingdom.