A primordial Orange Carotenoid Protein: Structure, photoswitching activity and evolutionary aspects.
Slonimskiy, Y.B., Zupnik, A.O., Varfolomeeva, L.A., Boyko, K.M., Maksimov, E.G., Sluchanko, N.N.(2022) Int J Biol Macromol 222: 167-180
- PubMed: 36165868
- DOI: https://doi.org/10.1016/j.ijbiomac.2022.09.131
- Primary Citation of Related Structures:
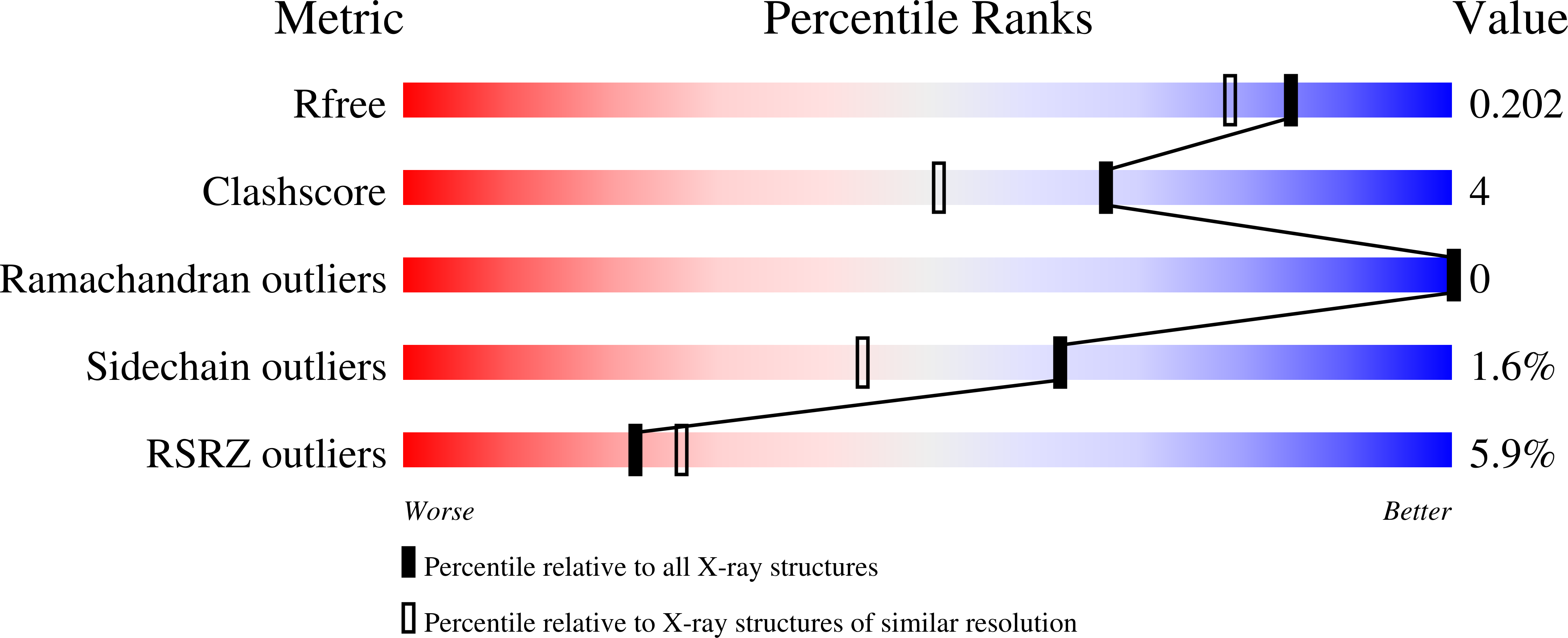
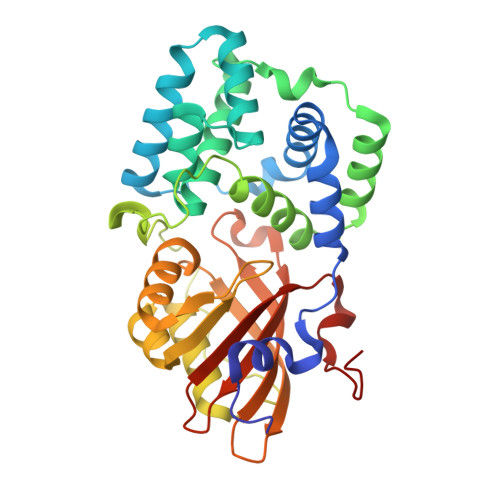
8A0H - PubMed Abstract:
Cyanobacteria are photosynthesizing prokaryotes responsible for the Great Oxygenation Event on Earth ~2.5?Ga?years ago. They use a specific photoprotective mechanism based on the 35-kDa photoactive Orange Carotenoid Protein (OCP), a promising target for developing novel optogenetic tools and for biomass engineering. The two-domain OCP presumably stems from domain fusion, yet the primitive thylakoid-less cyanobacteria Gloeobacter encodes a complete OCP. Its photosynthesis regulation lacks the so-called Fluorescence Recovery Protein (FRP), which in Synechocystis inhibits OCP-mediated phycobilisome fluorescence quenching, and Gloeobacter OCP belongs to the recently defined, heterogeneous clade OCPX (GlOCPX), the least characterized compared to OCP2 and especially OCP1 clades. Here, we describe the first crystal structure of OCPX, which explains unique functional adaptations of Gloeobacter OCPX compared to OCP1 from Synechocystis. We show that monomeric GlOCPX exploits a remarkable intramolecular locking mechanism stabilizing its dark-adapted state and exhibits drastically accelerated, less temperature-dependent recovery after photoactivation. While GlOCPX quenches Synechocystis phycobilisomes similar to Synechocystis OCP1, it evades interaction with and regulation by FRP from other species and likely uses alternative mechanisms for fluorescence recovery. This analysis of a primordial OCPX sheds light on its evolution, rationalizing renaming and subdivision of the OCPX clade into subclades - OCP3a, OCP3b, OCP3c.
Organizational Affiliation:
A.N. Bach Institute of Biochemistry, Federal Research Center of Biotechnology of the Russian Academy of Sciences, 119071 Moscow, Russian Federation.