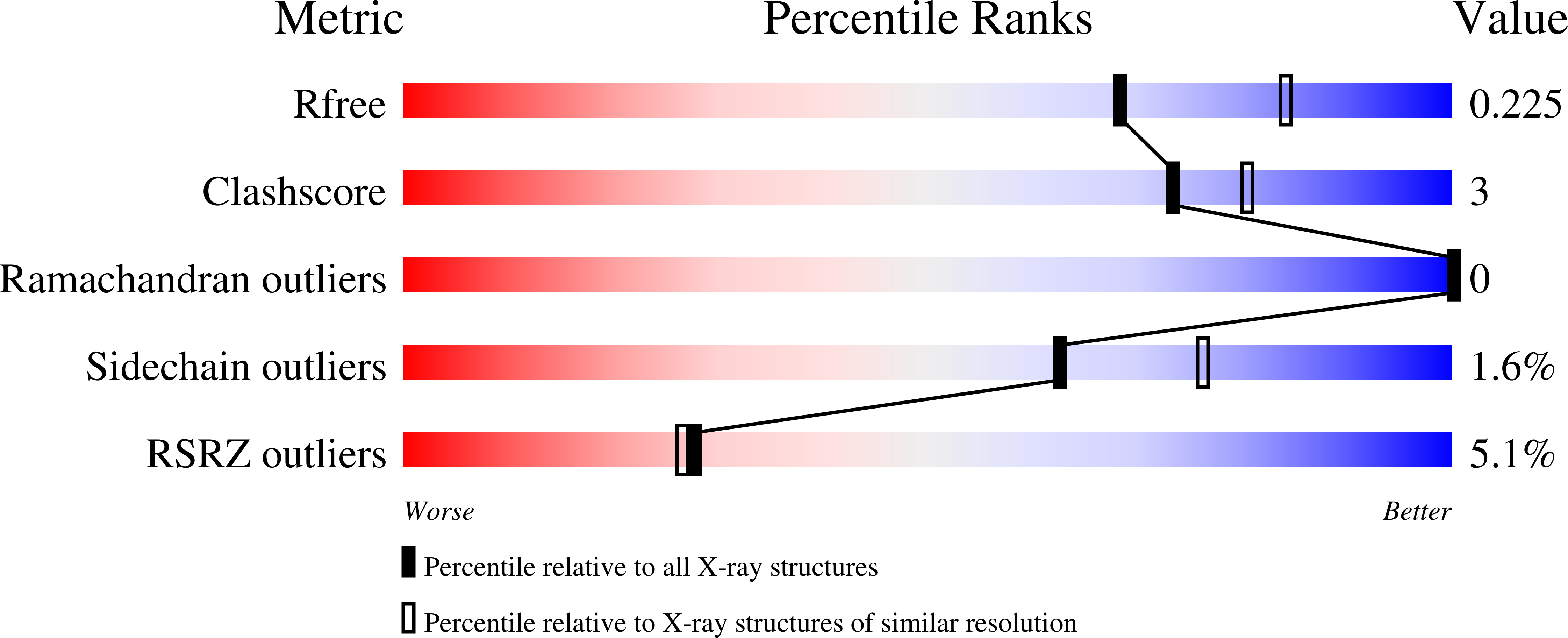
Bivalent molecular mimicry by ADP protects metal redox state and promotes coenzyme B 12 repair.
Gouda, H., Mascarenhas, R., Ruetz, M., Yaw, M., Banerjee, R.(2023) Proc Natl Acad Sci U S A 120: e2220677120-e2220677120
- PubMed: 36888659
- DOI: https://doi.org/10.1073/pnas.2220677120
- Primary Citation of Related Structures:
8DYJ, 8DYL - PubMed Abstract:
Control over transition metal redox state is essential for metalloprotein function and can be achieved via coordination chemistry and/or sequestration from bulk solvent. Human methylmalonyl-Coenzyme A (CoA) mutase (MCM) catalyzes the isomerization of methylmalonyl-CoA to succinyl-CoA using 5'-deoxyadenosylcobalamin (AdoCbl) as a metallocofactor. During catalysis, the occasional escape of the 5'-deoxyadenosine?(dAdo) moiety leaves the cob(II)alamin intermediate stranded and prone to hyperoxidation to hydroxocobalamin, which is recalcitrant to repair. In this study, we have identified the use of bivalent molecular mimicry by ADP, coopting the 5'-deoxyadenosine and diphosphate moieties in the cofactor and substrate, respectively, to protect against cob(II)alamin overoxidation on MCM. Crystallographic and electron paramagnetic resonance (EPR) data reveal that ADP exerts control over the metal oxidation state by inducing a conformational change that seals off solvent access, rather than by switching five-coordinate cob(II)alamin to the more air stable four-coordinate state. Subsequent binding of methylmalonyl-CoA (or CoA) promotes cob(II)alamin off-loading from MCM to adenosyltransferase for repair. This study identifies an unconventional strategy for controlling metal redox state by an abundant metabolite to plug active site access, which is key to preserving and recycling a rare, but essential, metal cofactor.
Organizational Affiliation:
Department of Biological Chemistry, University of Michigan Medical School, Ann Arbor, MI 48109.