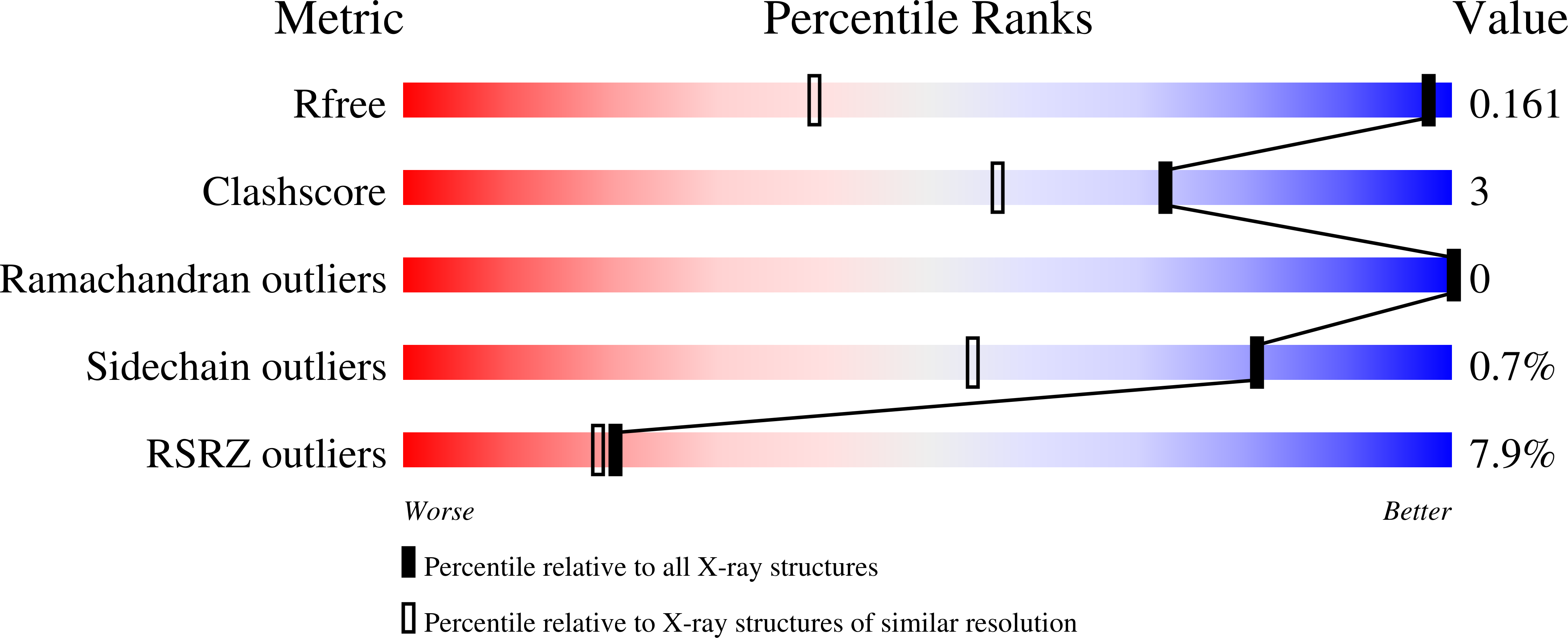
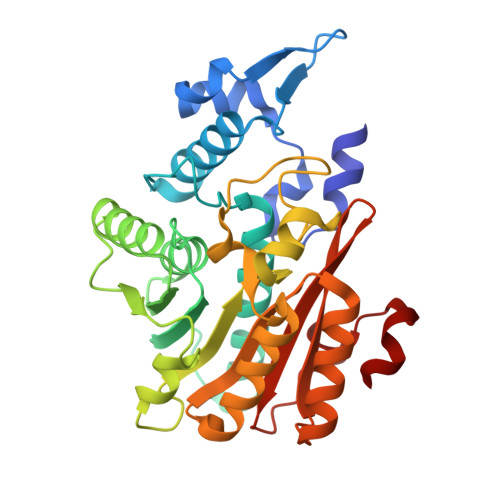
If you cannot see it, is it still there?
Leitner, L., Hudspeth, J., Werten, S., Rupp, B.(2025) J Appl Crystallogr 58: 615-621
- PubMed: 40170967
- DOI: https://doi.org/10.1107/S160057672500130X
- Primary Citation of Related Structures:
9GR6, 9GR7 - PubMed Abstract:
Protein crystallographers rely on electron density to build atomic models of molecular structures, yet flexible regions often remain unseen in electron density and are omitted. We suggest that ensemble refinement can be used to visualize and analyse the conformational landscape of such 'invisible' protein segments, which is particularly useful in cases where molecular flexibility plays a functional role. Using ensemble refinement on multiple crystal forms of the fungal methyl-transferase PsiM as an example, we illustrate the dynamic nature of a key substrate recognition loop, demonstrating its potential role in substrate binding and release. Ensemble refinement provides a persuasive visualization of biologically relevant flexible regions and can be a powerful tool for exploring molecular plasticity and aiding the modelling of dynamic protein components.
Organizational Affiliation:
Institute of Genetic Epidemiology Medical University of Innsbruck Sch?pfstrasse 41 6020Innsbruck Austria.