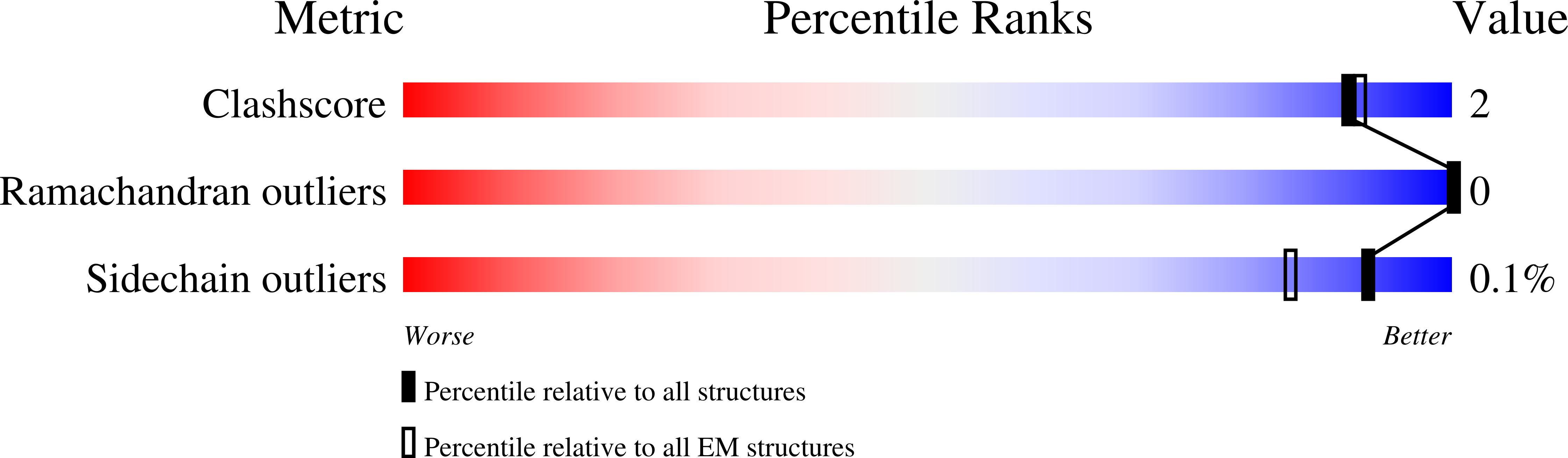Structural insights into subunit-dependent functional regulation in epithelial sodium channels.
Houser, A., Baconguis, I.(2025) Structure 33: 349-362.e4
- PubMed: 39667931
- DOI: https://doi.org/10.1016/j.str.2024.11.013
- Primary Citation of Related Structures:
9BLR, 9BTG, 9BTU - PubMed Abstract:
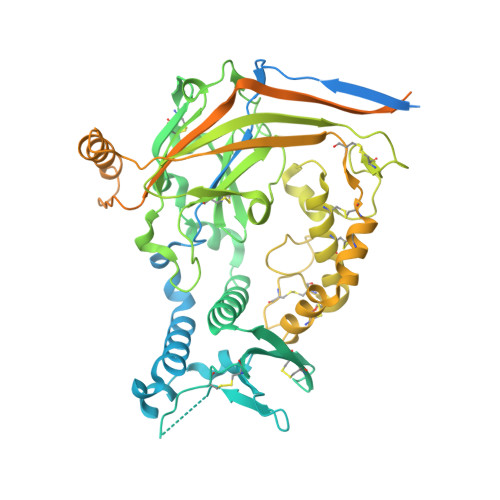
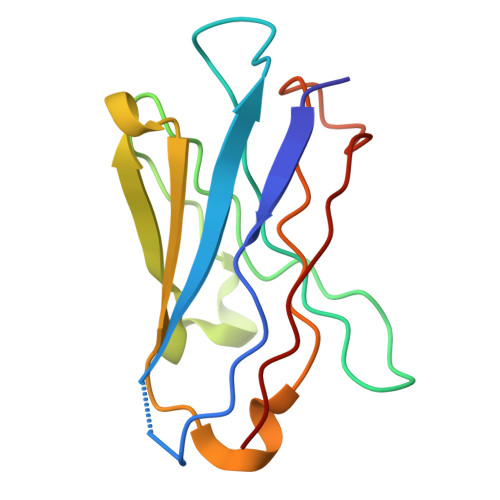
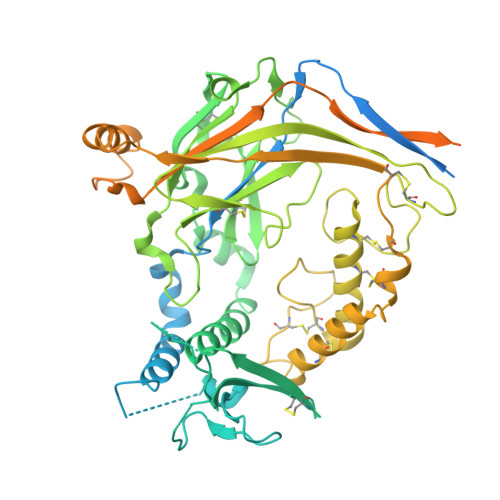
Epithelial sodium channels (ENaCs) play a crucial role in Na + reabsorption in mammals. To date, four subunits have been identified-¦Á, ¦Â, ¦Ã, and ¦Ä-believed to form different heteromeric complexes. Currently, only the structure of the ¦Á¦Â¦Ã complex is known. To investigate the formation of channels with different subunit compositions and to determine how each subunit contributes to distinct channel properties, we co-expressed human ¦Ä, ¦Â, and ¦Ã. Using single-particle cryoelectron microscopy, we observed three distinct ENaC complexes. The structures unveil a pattern in which ¦Â and ¦Ã positions are conserved among the different complexes while the ¦Á position in ¦Á¦Â¦Ã trimer is occupied by either ¦Ä or another ¦Â. The ¦Ä subunit induces structural rearrangements in the ¦Ã subunit, which may contribute to the differences in channel activity between ¦Á¦Â¦Ã and ¦Ä¦Â¦Ã channels. These structural changes provide molecular insights into how ENaC subunit composition modulates channel function.
Organizational Affiliation:
Neuroscience Graduate Program, Oregon Health & Science University, Portland, OR 97239, USA.