News
Extension to EMDB Accession Codes
06/25 
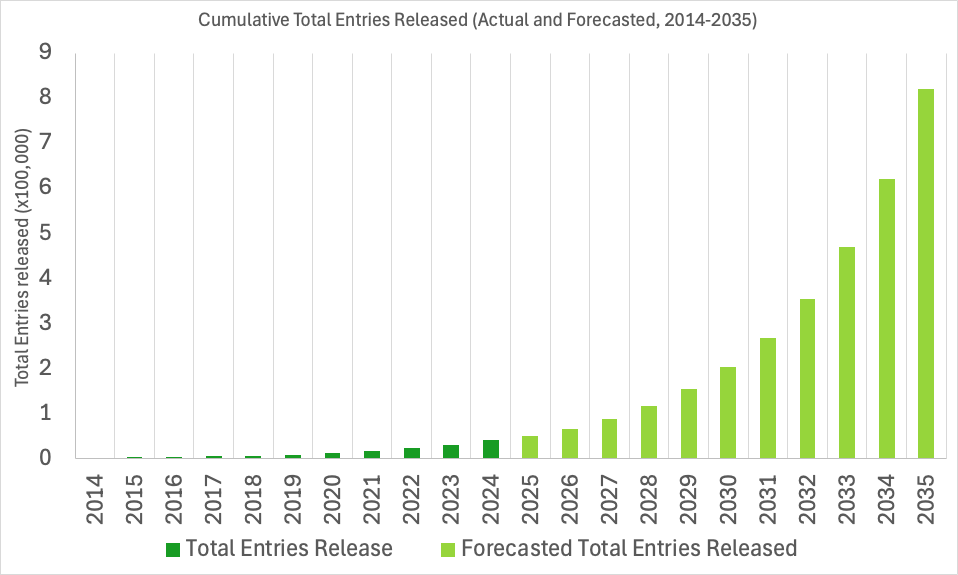
The EMDB archive is experiencing near-exponential growth. To support this rapid expansion, the wwPDB has been working to prepare our systems for the future and ensure seamless deposition, validation, and dissemination of data in the years ahead.
As part of this effort, we are announcing an important change:
EMDB IDs will be extended to support up to six digits, allowing for identifiers such as EMD-123456. Currently, the archive uses four and five-digit IDs (e.g., EMD-0123, EMD-45678), with support for identifiers up to the EMDB ID: EMD-99999. The change to six-digits will increase our capacity ten-fold and ensure ID availability into the next decade, up to EMD-999999.
We do not anticipate surpassing 99,999 released entries until around 2028, however, we expect to begin assigning six-digit EMDB IDs sometime in 2026. As such, as early as 2026, depositors should anticipate being assigned six-digit IDs within the wwPDB OneDep deposition system, and the user and developer community can expect six-digit EMDB IDs in the EMDB archive.
We appreciate your support as the archive grows and thank you for your contribution to the worldwide effort to archive structural biology data. We remain committed to ensuring a stable, sustainable and scalable infrastructure that supports the continued successes and productivity of the scientific community using structural biology and cryoEM in their research. For questions or feedback, contact the EMDB team at emdbhelp@ebi.ac.uk.
 EMDB Growth
EMDB Growth













