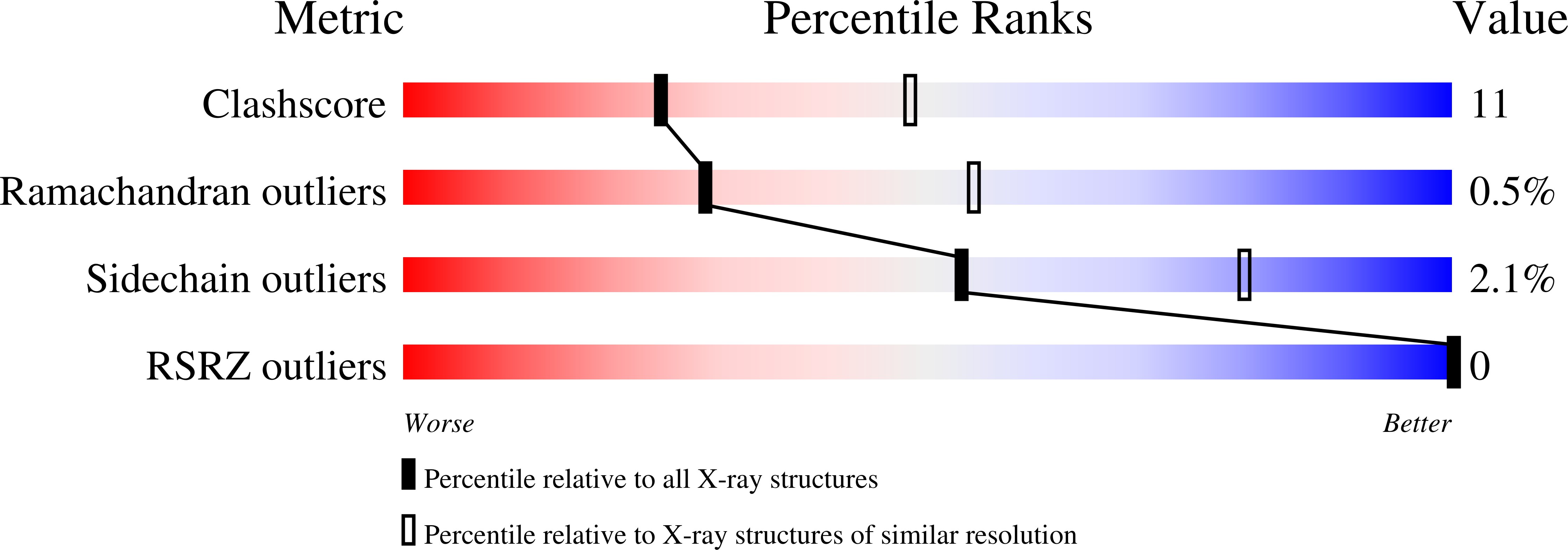
The role of glutamine 114 in old yellow enzyme
Brown, B.J., Hyun, J., Duvvuri, S.D., Karplus, P.A., Massey, V.(2002) J Biological Chem 277: 2138-2145
- PubMed: 11668181
- DOI: https://doi.org/10.1074/jbc.M108453200
- Primary Citation of Related Structures:
1K02, 1K03 - PubMed Abstract:
Glutamine 114 of OYE1 is a well conserved residue in the active site of the Old Yellow Enzyme family. It forms hydrogen bonds to the O2 and N3 of the flavoprotein prosthetic group, FMN. Glutamine 114 was mutated to asparagine, introducing an R-group that is one methylene group shorter. The resultant enzyme was characterized to determine the effect of the mutation on the mechanistic behavior of the enzyme, and the crystal structure was solved to determine the effect of the mutation on the structure of the protein. The Q114N mutation results in little change in the protein structure, moving the amide group of residue 114 out of H-bonding distance, allowing repositioning of the FMN prosthetic group to form new interactions that replace the lost H-bonds. The mutation decreases the ability to bind ligands, as all dissociation constants for substituted phenols are larger than for the wild type enzyme. The rate constant for the reductive half-reaction with beta-NADPH is slightly greater, whereas that for the oxidative half-reaction with 2-cyclohexenone is smaller than for the wild type enzyme. Oxidation with molecular oxygen is biphasic and involves formation and reaction with O(2), a phenomenon that is more pronounced with this mutation than with wild type enzyme. When superoxide dismutase is added to the reaction, we observe a single-phase reaction typical of the wild type enzyme. Turnover reactions using beta-NADPH with 2-cyclohexenone and molecular oxygen were studied to further characterize the mutant enzyme.
Organizational Affiliation:
Department of Biological Chemistry, University of Michigan, Ann Arbor, Michigan 48109-0606, USA.