The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment.
Bode, W., Mayr, I., Baumann, U., Huber, R., Stone, S.R., Hofsteenge, J.(1989) EMBO J 8: 3467-3475
- PubMed: 2583108
- DOI: https://doi.org/10.1002/j.1460-2075.1989.tb08511.x
- Primary Citation of Related Structures:
1PPB - PubMed Abstract:
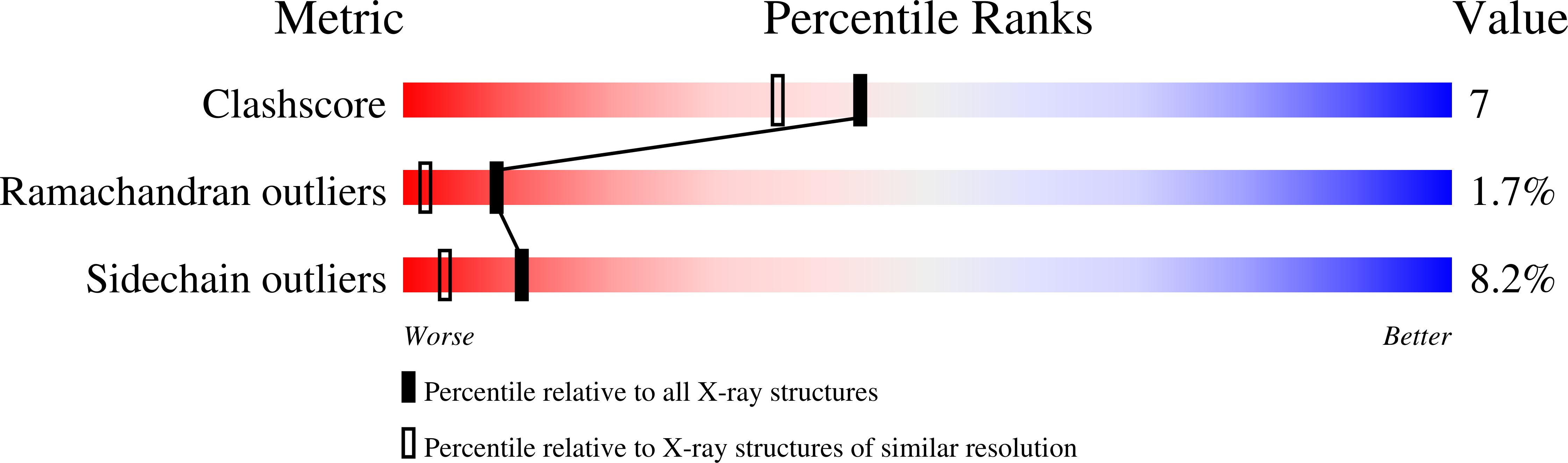
A stoichiometric complex formed between human alpha-thrombin and D-Phe-Pro-Arg chloromethylketone was crystallized in an orthorhombic crystal form. Orientation and position of a starting model derived from homologous modelling were determined by Patterson search methods. The thrombin model was completed in a cyclic modelling-crystallographic refinement procedure to a final R-value of 0.171 for X-ray data to 1.92 A. The structure is in full agreement with published cDNA sequence data. The A-chain, ordered only in its central part, is positioned along the molecular surface opposite to the active site. The B-chain exhibits the characteristic polypeptide fold of trypsin-like proteinases. Several extended insertions form, however, large protuberances; most important for interaction with macromolecular substrates is the characteristic thrombin loop around Tyr60A-Pro60B-Pro60C-Trp60D (chymotrypsinogen numbering) and the enlarged loop around the unique Trp148. The former considerably restricts the active site cleft and seems likely to be responsible for poor binding of most natural proteinase inhibitors to thrombin. The exceptional specificity of D-Phe-Pro-Arg chloromethylketone can be explained by a hydrophobic cage formed by Ile174, Trp215, Leu99, His57, Tyr60A and Trp60D. The narrow active site cleft, with a more polar base and hydrophobic rims, extends towards the arginine-rich surface of loop Lys70-Glu80 that probably represents part of the anionic binding region for hirudin and fibrinogen.
Organizational Affiliation:
Max-Planck-Institut f¨¹r Biochemie, Martinsried, FRG.