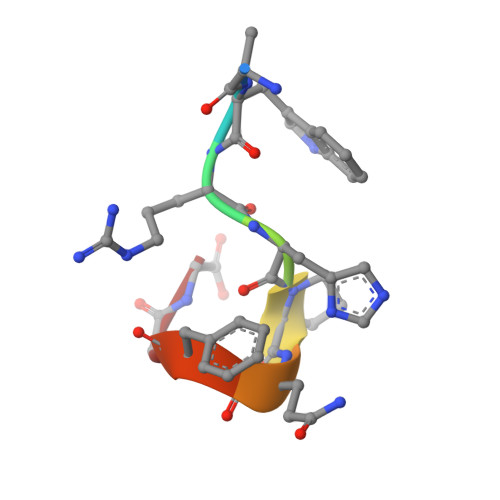
Molecular interaction between the Strep-tag affinity peptide and its cognate target, streptavidin.
Schmidt, T.G., Koepke, J., Frank, R., Skerra, A.(1996) J Mol Biology 255: 753-766
- PubMed: 8636976
- DOI: https://doi.org/10.1006/jmbi.1996.0061
- Primary Citation of Related Structures:
1RST, 1RSU - PubMed Abstract:
The Strep-tag is a selected nine-amino acid peptide (AWRHPQFGG) that displays intrinsic binding affinity towards streptavidin and has been used as an affinity tag for recombinant proteins. In order to elucidate the molecular mechanism underlying this type of artificial protein-peptide recognition, X-ray crystallographic analyses and binding measurements were carried out. The crystal structure of the complex between recombinant core streptavidin and the synthesized peptide was solved and refined at 1.7 A resolution (space group I4(1)22; unit cell dimensions a = b = 58.3 A, c = 176.9 A). The Strep-tag was bound at the same surface pocket where biotin, the natural ligand of streptavidin, gets complexed. The peptide backbone exhibited 3(10)-helical conformation, with eight of the residues involved in protein contacts. The C-terminal Gly-Gly moiety of the Strep-tag participated in a salt bridge to Arg84 of streptavidin with its free carboxylate group. This finding explained why the use of the Strep-tag in fusions with recombinant proteins was restricted to their carboxyl end. Employing a synthetic peptide spot assay, the variant Strep-tag II was screened, which did not have this limitation. The isomorphous crystal structure of its complex with streptavidin revealed that a glutamate side-chain provided the salt bridge in this case, with an otherwise almost unchanged mode of binding. Affinity constants between the peptides and streptavidin were measured by isothermal titration calorimetry. A value of 2.7 x 10(4) M-1 was determined for the Strep-tag peptide, and slightly tighter binding was seen when the Strep-tag was applied as part of a bacterially produced fusion protein. This affinity is significantly higher, compared with values previously reported for shorter streptavidin-binding peptides, and agrees well with the remarkable selectivity observed in recombinant protein purification applications.
Organizational Affiliation:
Max-Planck-Institut f¨¹r Biophysik, Frankfurt/Main, Germany.