Design, Synthesis, and Structural Analysis of Phenylpropanoic Acid-Type PPAR gamma-Selective Agonists: Discovery of Reversed Stereochemistry-Activity Relationship
Ohashi, M., Oyama, T., Nakagome, I., Satoh, M., Nishio, Y., Nobusada, H., Hirono, S., Morikawa, K., Hashimoto, Y., Miyachi, H.(2011) J Med Chem 54: 331-341
- PubMed: 21128600
- DOI: https://doi.org/10.1021/jm101233f
- Primary Citation of Related Structures:
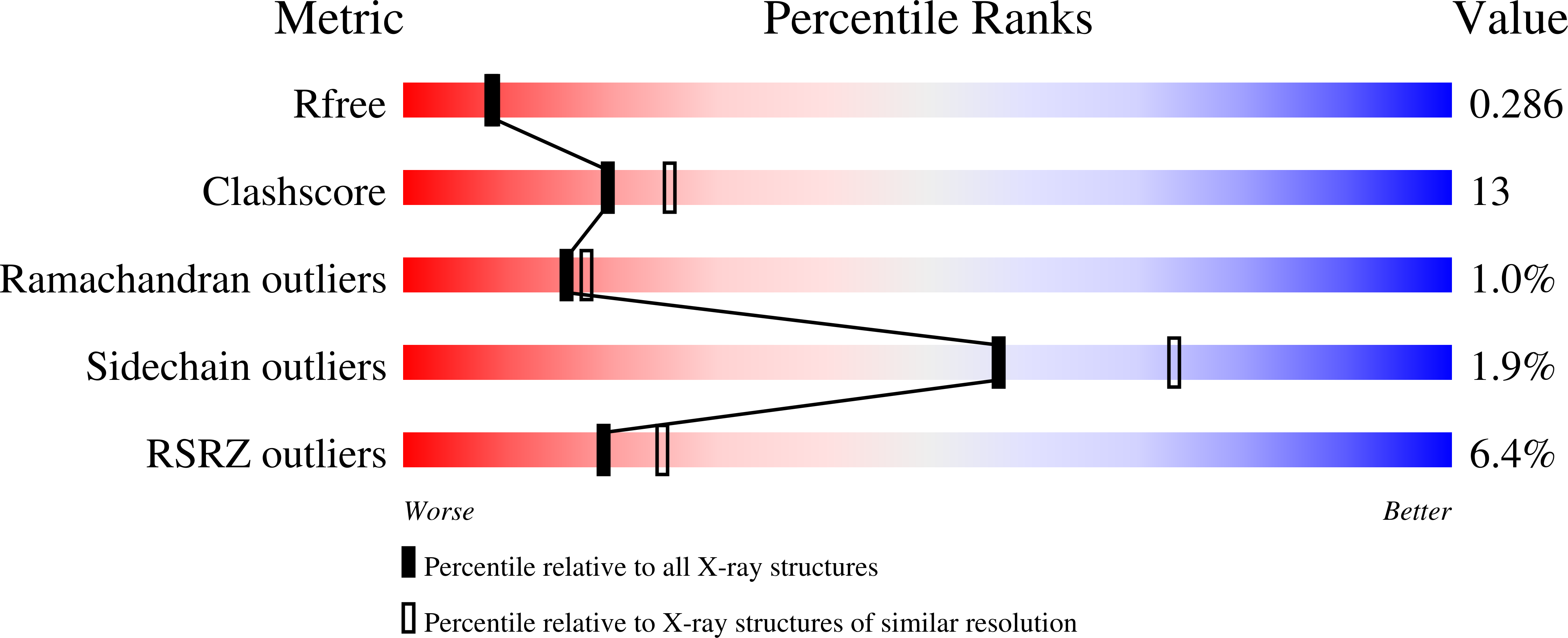
3AN3, 3AN4 - PubMed Abstract:
Peroxisome proliferator-activated receptor gamma (PPAR¦Ã) is a ligand-mediated transcription factor with roles in glucose, lipid, and lipoprotein homeostasis, and PPAR¦Ã ligands are expected have therapeutic potential in these as well as other areas. We report here the design, synthesis, crystallographic analysis, and computational studies of ¦Á-benzylphenylpropanoic acid PPAR¦Ã agonists. Interestingly, these compounds show a reversal of the stereochemistry-transactivation activity relationship observed with other phenylpropanoic acid ligands.
Organizational Affiliation:
Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Kita-ku, Okayama, Japan.