TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists.
Menuz, K., Stroud, R.M., Nicoll, R.A., Hays, F.A.(2007) Science 318: 815-817
- PubMed: 17975069
- DOI: https://doi.org/10.1126/science.1146317
- Primary Citation of Related Structures:
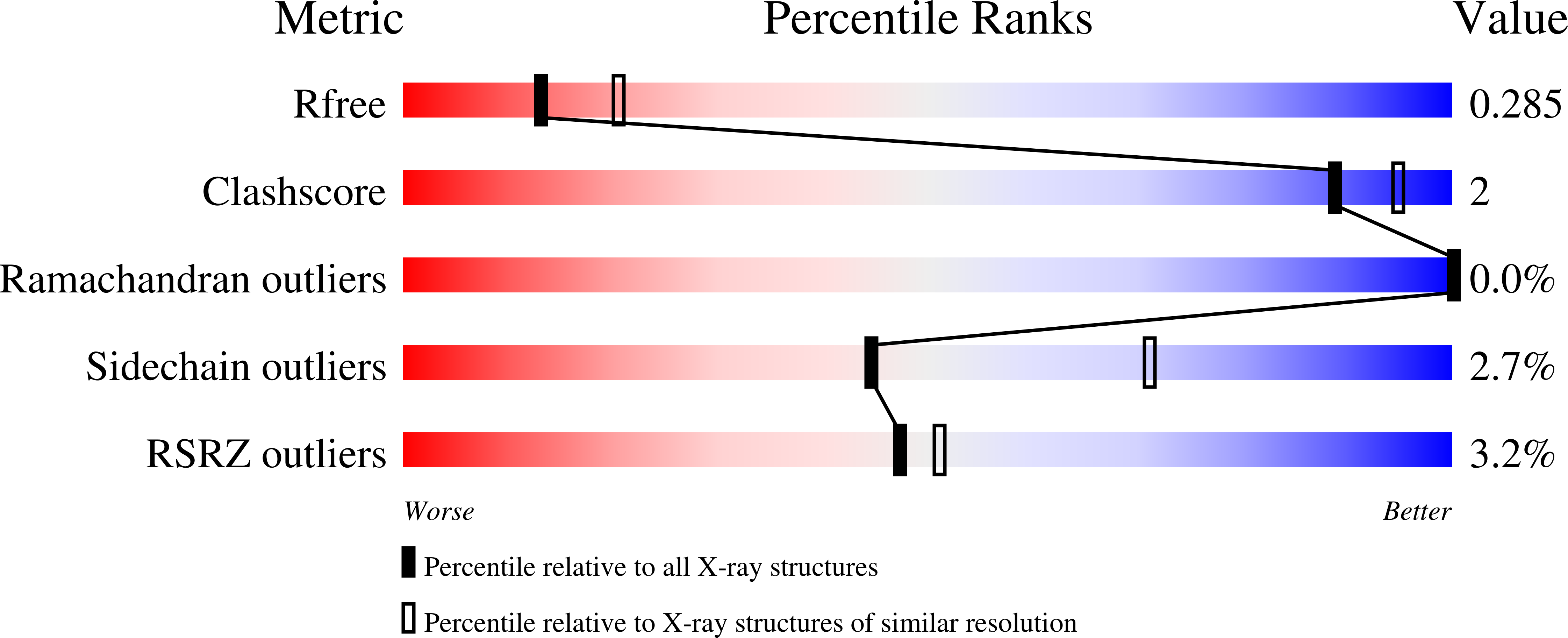
3B7D - PubMed Abstract:
Quinoxalinedione compounds such as 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) are the most commonly used alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonists. However, we find that in the presence of transmembrane AMPA receptor regulatory proteins (TARPs), which are AMPA receptor auxiliary subunits, CNQX acts as a partial agonist. CNQX induced small depolarizing currents in neurons of the central nervous system, and reconstitution of this agonist activity required coexpression of TARPs. A crystal structure of CNQX bound to the TARP-less AMPA receptor ligand-binding domain showed that, although CNQX induces partial domain closure, this movement is not transduced into linker separation, suggesting that TARPs may increase agonist efficacy by strengthening the coupling between domain closure and channel opening. Our results demonstrate that the presence of an auxiliary subunit can determine whether a compound functions as an agonist or antagonist.
Organizational Affiliation:
Department of Cellular and Molecular Pharmacology, University of California at San Francisco, San Francisco, CA 94143, USA.