Molecular Bases for the Recognition of Short Peptide Substrates and Cysteine-Directed Modifications of Human Insulin-Degrading Enzyme
Malito, E., Ralat, L.A., Manolopoulou, M., Tsay, J.L., Wadlington, N.L., Tang, W.J.(2008) Biochemistry 47: 12822-12834
- PubMed: 18986166
- DOI: https://doi.org/10.1021/bi801192h
- Primary Citation of Related Structures:
3CWW - PubMed Abstract:
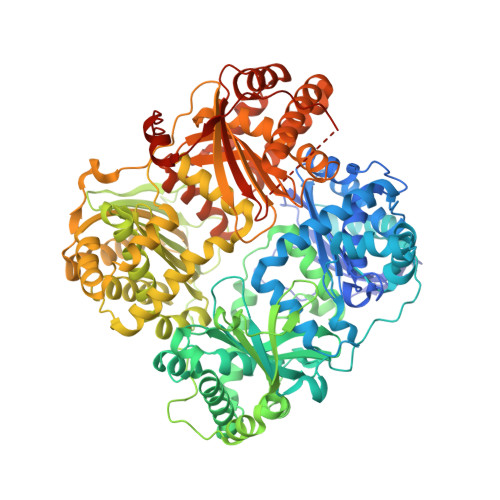
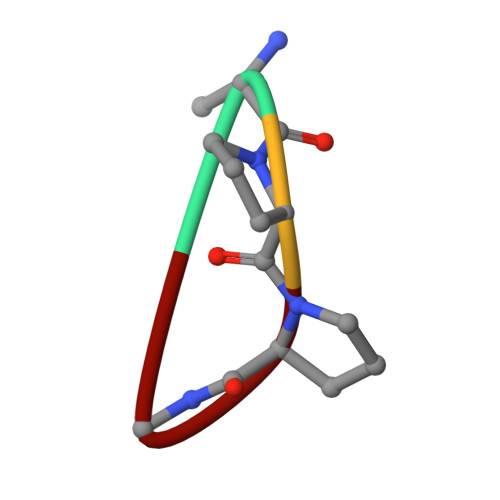
Insulin degrading enzyme (IDE) utilizes a large catalytic chamber to selectively bind and degrade peptide substrates such as insulin and amyloid beta (Abeta). Tight interactions with substrates occur at an exosite located approximately 30 A away from the catalytic center that anchors the N-terminus of substrates to facilitate binding and subsequent cleavages at the catalytic site. However, IDE also degrades peptide substrates that are too short to occupy both the catalytic site and the exosite simultaneously. Here, we use kinins as a model system to address the kinetics and regulation of human IDE with short peptides. IDE specifically degrades bradykinin and kallidin at the Pro/Phe site. A 1.9 A crystal structure of bradykinin-bound IDE reveals the binding of bradykinin to the exosite and not to the catalytic site. In agreement with observed high K(m) values, this suggests low affinity of bradykinin for IDE. This structure also provides the molecular basis on how the binding of short peptides at the exosite could regulate substrate recognition. We also found that human IDE is potently inhibited by physiologically relevant concentrations of S-nitrosylation and oxidation agents. Cysteine-directed modifications play a key role, since an IDE mutant devoid of all 13 cysteines is insensitive to the inhibition by S-nitrosoglutathione, hydrogen peroxide, or N-ethylmaleimide. Specifically, cysteine 819 of human IDE is located inside the catalytic chamber pointing toward an extended hydrophobic pocket and is critical for the inactivation. Thiol-directed modification of this residue likely causes local structural perturbation to reduce substrate binding and catalysis.
Organizational Affiliation:
Ben-May Department for Cancer Research, Biological Science Collegiate Division, and Committee on Neurobiology, The University of Chicago, Chicago, Illinois 60637, USA.